Ultrasensitive Lateral Flow Immunochromatography for Low-Cost Cancer Screening
Background and Significance. Although cervical cancer is a preventable and/or curable disease if detected early, it continues to claim the lives of many relatively young women, especially in low middle-income countries (LMICs) [1, 2]. Cervical cancer is the fourth most common cancer in women after breast, colorectal, and lung cancers) and seventh overall. Globally in 2020, an estimated 604,127 cervical cancer cases and 341,831 deaths were noted (age-standardized incidence is 13.3 cases per 100,000 women [3]. The American Cancer Society estimates (2023) about 13,960 new cases will be diagnosed in the United States annually and 4,310 women will die from it. In Vietnam, 4,132 women are diagnosed and 2,223 die from Cervical cancer [1]. Cytology-based screening (Papanicolaou smear screening) has significantly reduced cervical cancer mortality rates in high-income countries [4, 5], but cannot be implemented in low-resource settings [6, 7]. To overcome the limitations of cytology-based screening programs in LMICs, two alternative tests have been examined: visual inspection with acetic acid (VIA) and Human Papillomavirus (HPV) tests. VIA is an inexpensive, simple point-of-care (POC) test that can be performed by mid-level clinical providers. This test is because precancerous tissue (called acetowhite lesions) turns white when 5% acetic acid (household vinegar) is applied to the cervical epithelium, while healthy tissue remains pink. However, VIA only has moderate sensitivity and specificity (~60%) and its precursors [8] and test performance declines significantly in older women [9]. The more general HPV DNA or RNA PCR tests for cervical cancer screening, are based on the fact that infection with high-risk HPV (hrHPV) is required to induce the molecular transformations that lead to precancer and cancer [10]. Many versions of HPV DNA (genotypes 16, 18, 31, 58) and RNA tests are now commercially available. Although highly sensitive (94% for Hybrid Capture 2; 96% for APTIMA), their specificity for cervical intraepithelial neoplasia grade 2 and 3 or worse (CIN2/3+), is modest (38% for Hybrid Capture 2; ~43% for APTIMA) [11]. Given its moderate specificity and positive predictive value, a positive HPV test does not imply that medical intervention is necessary to treat CIN2/3+ in many cases. Indiscriminate treatment of HPV-positive women may lead to overtreatment, side effects, emotional distress, and unnecessary cost. This complexity suggests a clear need for better (or perhaps multiple) diagnostic options that report on relevant clinical markers in a quantitative manner to make intelligent decisions [2].
Innovation. The major goal of our VinUni-Illinois Smart Health Center (VISHC) application is to develop a point-of-care (POC) technology for protein and DNA/RNA monitoring based on colorimetry that can deliver ultrahigh sensitivity utilizing the lateral flow platform and machine learning tools. Lateral flow (LF) utilizes the driving force of the liquid to present the analytes at the signal generation site and has been integrated with other detection modalities for highly sensitive detection of disease targets, including cervical cancer protein and nucleic acid targets [13-15]. The world market for LF-based tests was estimated as $20.5 billion in 2022 and expected to reach $22.6 billion by 2027. Due to COVID-19, the general population is much more familiar with LF-based tests, and there is significant infrastructure to manufacture and distribute these LF-based tests all over the world [16].While the technology exists, future efforts in this technology-market space need to address sensitivity, reproducibility, quantification, multiplexing, and cross-platform detection capability (antibody-based and nucleic acid-based). Lateral flow immunochromatography (LF-IC) kits are one of the most used POC assays in the market to date because of its low cost and simplicity.
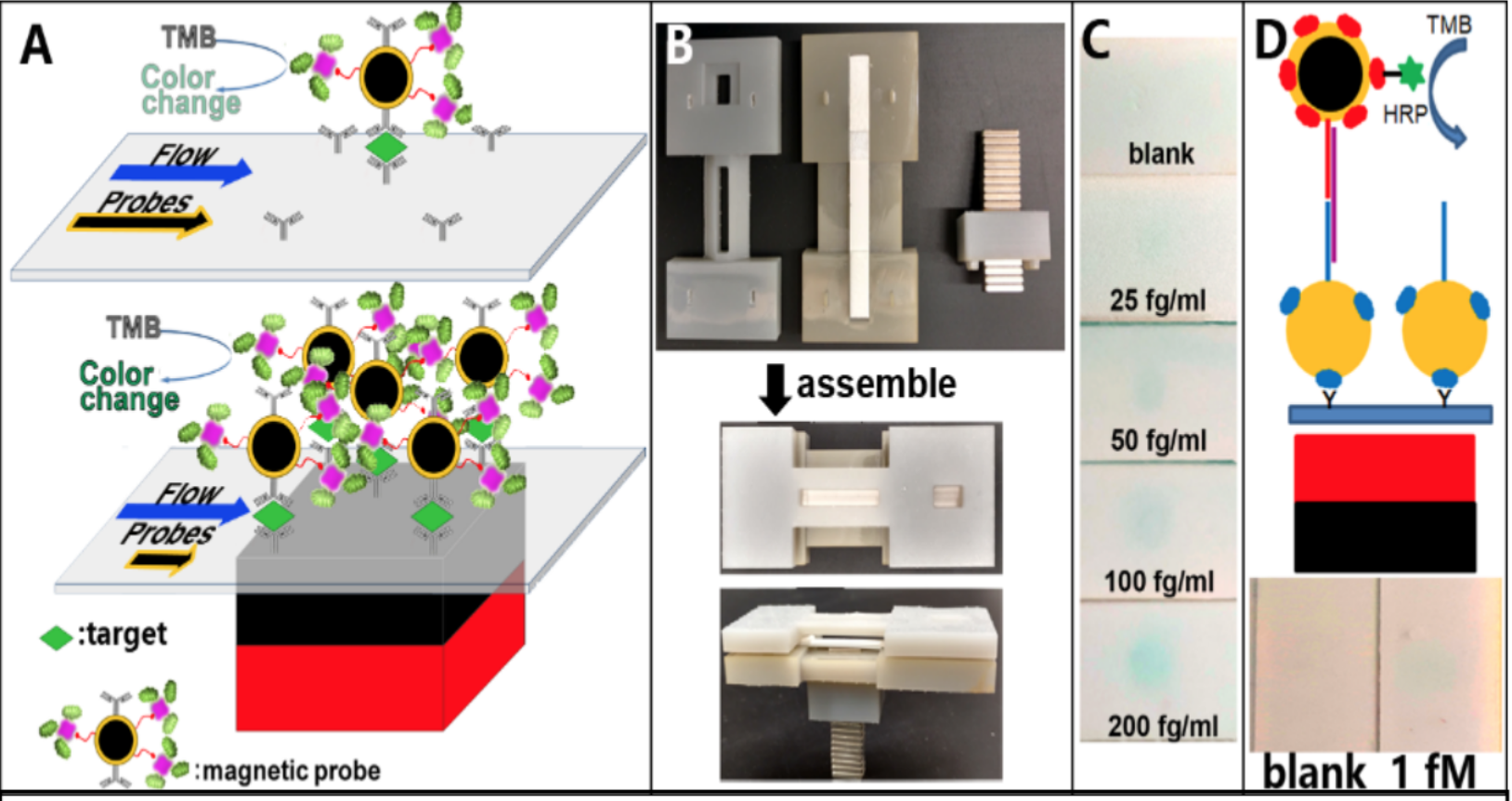
Figure 1. Schematic of magnetic focus enhancement compared with conventional LFA (A). Initial design prepared with 3D printing (B), to detect cervical cancer markers (C) and DNA sequences (D) without amplification steps utilizing RNA from E. coli O157:H7.
In our initial studies, we have shown that an unprecedented LOD of 25 fg/ml (Fig. 1C) of cervical cancer biomarkers can be achieved by increasing the capture ratio of the target at the signal generation site using a simple magnet (Fig. 1) [12,13]. This is the lowest reported limit by colorimetry to date.
Here we will reverse engineer our devise to elucidate the underlying engineering principles and mechanisms involved in the development of a POC magnetic focus enhanced LF-IC (mLF-IC) prototype for routine screening incorporating intelligent image analysis on commonly available smartphones. Innovative attributes are: 1) understand and quantitate the flow fields in the LF devise with particle image velocimetry (PIV) and/or darkfield microscopy at single nanoparticle sensitivity, 2) achieve a LOD in the fg/ml range for protein and RNA biomarkers of cervical cancer with >95% sensitivity and > 95% specificity, 3) detection by colorimetry utilizing intelligent image processing algorithms, 4) potential to multiplex, 5) extension of the technology to detect infectious pathogens for health and infectious disease and food safety screening to detect as low as 10 cfu/ml is possible (our prior work has shown a LOD of 25 cfu/ml by colorimetry, [15]). Our multidisciplinary approach will have the potential to transform POC analysis to single molecule regime, allowing monitoring and quantification of protein and DNA/RNA biomarker targets at an unprecedented level by colorimetric approach by integrating intelligent image analysis modules through this partnership.
Approach. In LF-IC, sample applied on the sample application pad flows through the LF-IC nitrocellulose membrane strip by capillary action and reacts with the pre-immobilized reagents (nanoparticle probes and signal enhancing labels) at the conjugate pad and captured at the signal generation site (or capture site) to provide a signal (Fig 1A). A key limitation is the lack of control of the movement of molecules once the sample is applied on the strip, limiting the reproducibility of results. Most of the conventional LF-IC assays use gold nanoparticles, limiting its sensitivity but maintains its simplicity. Reads outs based on magnetism, fluorescence, Raman/IR spectroscopy, luminescence, and electrochemistry exists spanning a range of applications. Other attempts were made to increase the interaction time between targets and capture probes to achieve an enhanced signal using different detection modalities/readouts. Another means to increase LOD is to slow down the movement of targets in the LF strip to allow for a prolonged residence time at the signal generation region, by allowing for a longer reaction/binding time between targets and capture ligands utilizing wax pillars, however improvement in LOD was not significant and a heater was required to melt the wax [17]. By integrating an enzyme amplification step with a magnetic focus concept, in our recent work we have shown that a 103-104 fold improvement in LOD can be achieved, possibly due to a higher capture efficiency enabled by a magnet at the signal generation site [15]. This approach retained the simplicity of the LF-IC unit enabling detection of infectious pathogens at an unprecedented LOD of 25 cfu/ml (conventional limit is 104 cfu/ml). Development of a POC technology by colorimetry that rivals the sensitivity of fluorescence, or other reader-based devices is attractive not only for cancer but also for a myriad of health screening applications in low resource settings.
Preliminary Work
A) Development and optimization of nanoparticle conjugates. Based on our previous work, two key evaluations need to occur for optimizing the nanoparticle (NP) probes in mLF-IC. Since the signals obtained from the immunoreaction are directly proportional to the amount of enzyme (i.e., Horseradish peroxidase, HRP) participating in the reaction, it is crucial to tether as many HRP molecules to the AuFe nanoparticle (NP) surface. Two conjugates are designed for this purpose: Conjugate I (biotinylated AuFe probe with target capture antibodies) and Conjugate II(streptavidin-HRP). The number of (c) SA-HRP and antibody functionalized AuFe104-fold improvement in LOD can be achieved by colorimetry and we will adopt this probe for signal generation with a magnet approach to develop calibration standards and quantification protocols with intelligent image analysis modules.
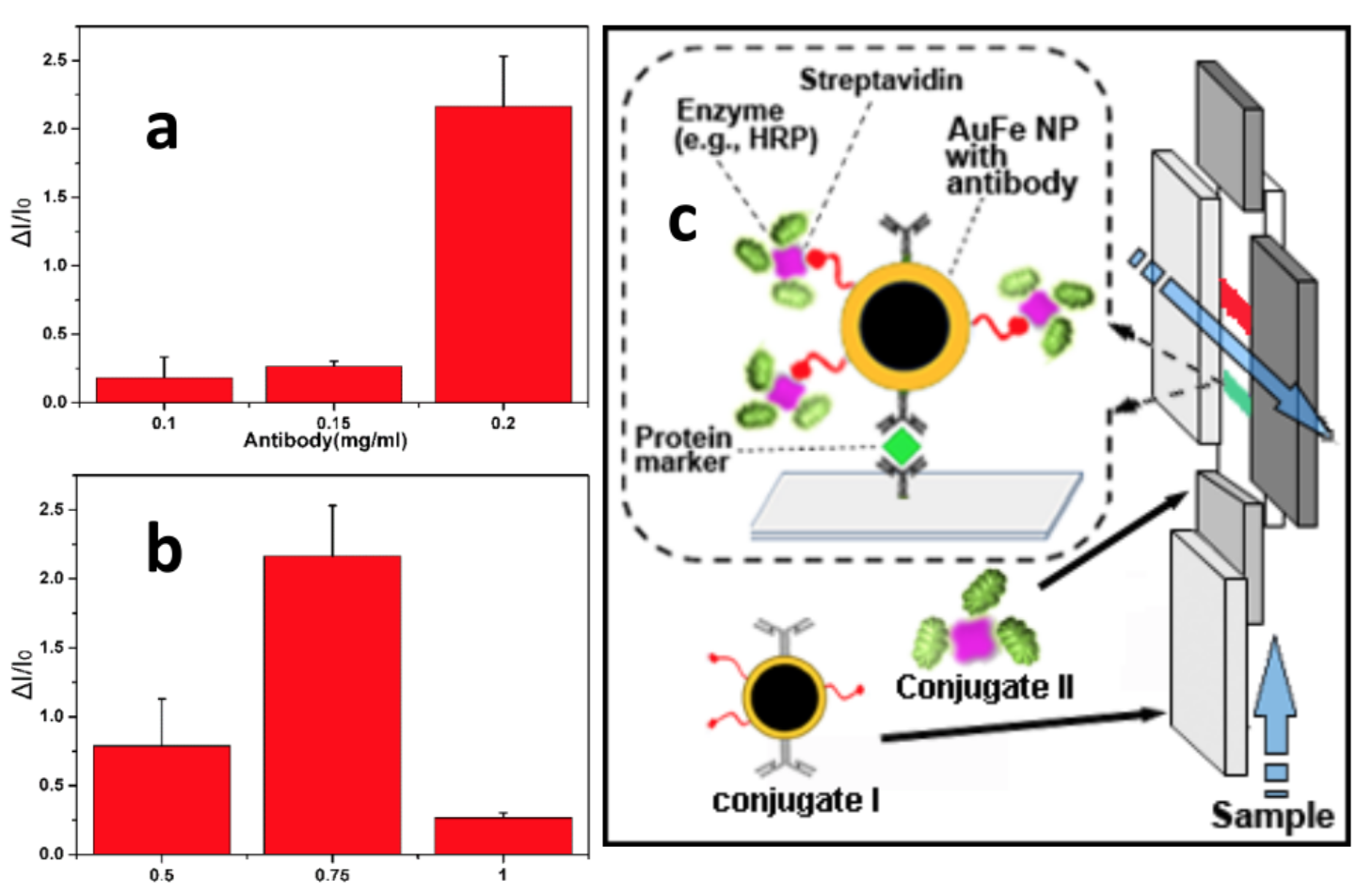
Figure 2. Optimization of (a) antibody concentration and (b) HRP at in the AuFe probe. (c) SA-HRP and antibody functionalized AuFe probe for signal generation with a magnet.
B) Evaluation of cervical cancer biomarkers. We will use established markers, our previous work [12] and others (Table 1), to complement the HPV cervical cancer screening. Commercially available cervical cancer in the market is from Biomerieux, Roche, Qiagen, CerMed Corp., Gen-Probe, among others. The kits are based on HPV (DNA/RNA) and has poor specificity (40-60%) (Table 1, rows 3 & 4). Other biomarkers (Table 1, rows 1 & 2) have also been evaluated and compared to the APTIMA HPV test (Table 1, row 3) and other HPV DNA tests (Table 1, row 4) [References provided upon request].
Based on our preliminary work over the past five years, we have validated a key cervical cancer biomarker, Valosin Containing Protein (VCP) and confirmed its expression in cervical tissue samples (n=200; normal, CIN1, CIN2, CIN3, and carcinoma) as detailed in work [12]. VCP is known to be involved in the ubiquitin/proteasome degradation pathway, which works in both up-regulation of cell proliferation and down-regulation of cell death in human cancer cells. Evidence from analyses of large patient cohorts demonstrated that significant increase in expression of VCP in tumor cells often correlate with disease progression [18,19] and VCP over-expression was found to be linked directly to hrHPV mediated activation of protein tyrosine phosphatases, non-receptor type (PTPNs) [20-22]. VCP identified CIN2, CIN3, and carcinoma (invasive cancer) with high sensitivity (99%) and specificity (95%) [13].
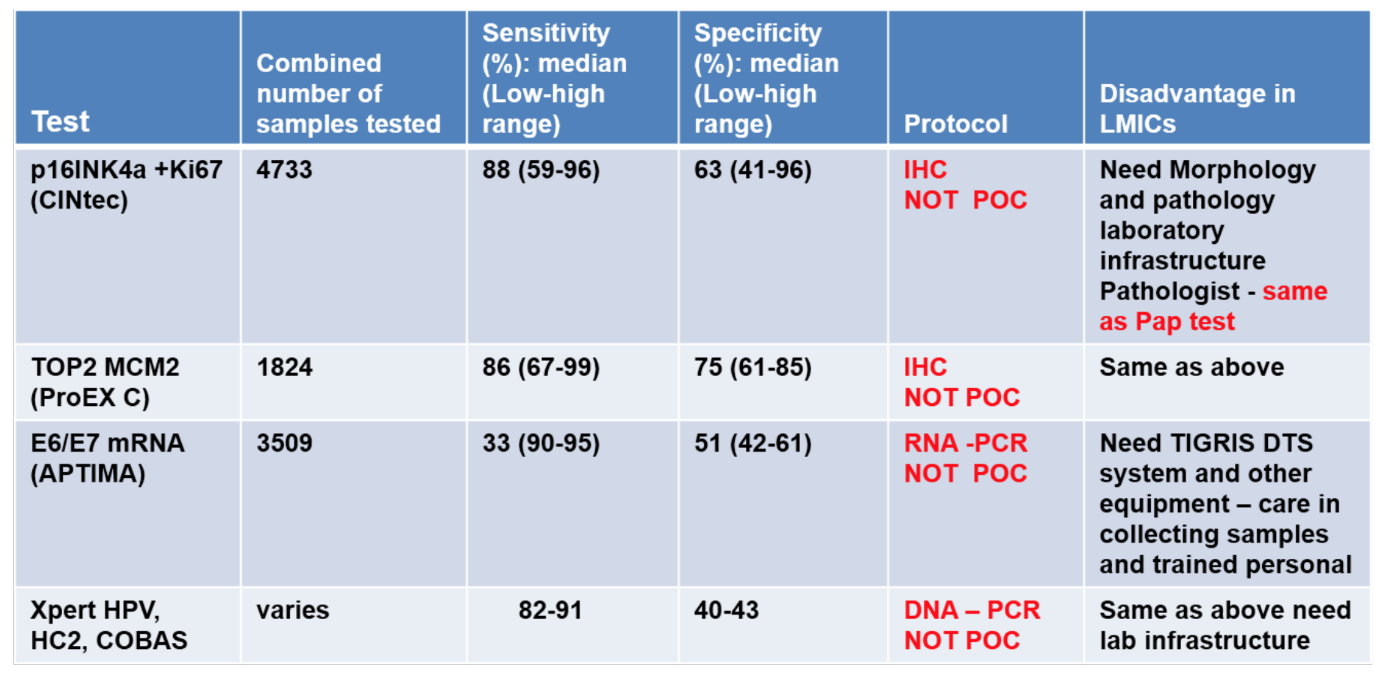
Table 1. Evaluation of commercial tests to detect p16INK4a, MCM2, TOP2 compared to HPV
In addition to VCP, to demonstrate multiplexing we will evaluate p16INK4a (cyclin dependent kinase-inhibitor 2A), which has been accepted as a sensitive and specific marker of dysplastic cells of the cervix and used in cervical screening [23]. A diagnostic test, CINtec Plus, by MTM laboratories AG (Heidelberg, Germany) has been formulated using an immunohistochemistry-based P16INK4a and Ki-67 antibody cocktail. In Europe, for the European Equivocal or Mildly Abnormal Pap Cytology Study (EEMAPS), the sensitivity of the CINtec test for biopsy-confirmed CIN2+ was 92% for atypical cells of undetermined significance cases and 94% for low-grade squamous intraepithelial lesions, with specificity of 81% and 68%, respectively. In addition, a p16INK4a ELISA assay detected CIN3 with an estimated sensitivity in the range of 80–95%, which is comparable to the HPV DNA test (Hybrid Capture 2). The p16INK4a ELISA test was more specific compared to the Hybrid Capture 2 test and was designed to either complement cytology or be ELISA-based. Hence, it cannot be performed as a point of care. Details of these markers and their relevance to cervical cancer screening based on patient samples are provided in our prior work [12].
C) Detection of VCP by mLF-IC. We have shown that with the magnetic focus enhanced LF-IC (mLF-IC) concept we can detect VCP protein target at a LOD of 25 fg/ml (conventional limit is in ng/ml or at best in pg/ml range). Utilizing enhancement strategies [14,19] and a simple magnet at the signal generation site, we expect to enhance the LOD of conventional LF-IC units by ~104 fold and develop a suitable quantification protocol, which is a gap in lateral flow technology. The expect the detection time to be within 45 minutes based on our preliminary work. Fig. 1C shows a gradation of images based on VCP levels detected by the mLF-IC method. The expected clinical sensitivity will be >90% with an unparalleled LOD (25 fg/ml). In addition to VCP, we have also evaluated the LOD (100 fg/ml) for P16INK4a (Fig. 3).
Methods corresponding to Specific Aims
Specific Aim 1. Reverse engineer the design and development of mLF-IC technology to achieve fg/ml LOD and quantitative cervical cancer POC screening.
A) Capturing Ligands. Antibodies and Aptamers: (i) Antibodies for the markers discussed are commercially obtained from Abcam (VCP: Anti-VCP antibody Cat# ab11433, p16INK4a: Anti- p16INK4a antibody Cat # ab54210). Antibodies for other comparative tests with MCM2 and TOP2A in Table 1 (MCM2: Anti-MCM2 antibody Cat# ab108935, and TOP2A: Anti-Topoisomerase II alpha antibody Cat# ab52934) are also available. Alternatively, aptamers specific to the proteins of interest can also be fabricated (Prior discussions with Base Pair Biotechnologies Inc., an established manufacturer with significant experience in collaborating with NIH funded investigators). Purified proteins will be obtained from Novus Biologicals, LLC.
B) mLF-IC Technology Development. The sample containing the target protein will be applied at the sample pad of the immunostrip to initiate a flow in the longitudinal direction to induce immunocomplex formation at a signal generation site where the capture antibody is immobilized (Fig. 1a). During this flow, several SA-HRP conjugates can be coupled to the branched biotin on conjugate I, to create an enzyme-network that will be enhanced by the tetramethyl benzidine (TMB) interaction at the substrate supply pad (Fig. 1a,b) to initiate the enzymatic signal generation. The magnet can be removed after the immunocomplex formation (5-10 min after application of sample). Since LF-IC detection is based on capillary flow, we will test that hypothesis that by reducing the movement of the target and conjugates in the strip with the magnet, the capture efficiency can be increased (a constraint in the current LF technologies) to attain a 104-fold increase in LOD (Fig. 1c,d). We have previously shown the reduced mobility of the conjugates by particle image velocimetry [13].
C) Evaluation of performance metrics. We hypothesize that the capture ratio of target protein in a LF devise is one of the key factors that determine detection sensitivity. To evaluate flow fields and to assess the target capture ratio, we will utilize 6-8 different concentrations in the range between 25 fg/ml – 500 ng/ml (25 fg/ml, 250 fg/ml, 500 fg/ml, 100 pg/ml, 500 pg/ml, 1 ng/ml, 250 ng/ml, 500 ng/ml). The capture ratio at the signal generation site will be evaluated by the following:
Evaluation of LOD: We will assess the LOD by evaluating the proteins (VCP and p16INK4a) in the concentration range. HPV will be used for control in all experiments. Quantity of probes captured at the signal generation site will be assessed by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and increased capture efficiency by mLF-IC will be estimated.
Standard assays for quantification. We will utilize ELISA when possible and mass spectrometry for ultra-low concentrations [13] to assess the initial target levels for calibration purposes. A smartphone with intelligent image processing app that can capture LF image, detect the signal generation sites, measure the colorimetry of these sites, convert them precisely to the target concentration, and infer the cancer screening results. The smartphone app will also upload the captured images and extracted results to a cloud for large-scale calibration and analytics by machine learning.
D) Deliverables. Upon completion of Aim 1, we will know the enhanced capture ratio at the signal generation site that will allow ultralow LOD. We will also standardize the concentration of capture antibodies at the target site, and the AuFe probes for the assay. All experiments will be conducted in replicates of three and tested at 0.05 significance level.
E) Pitfalls and Alternatives. Alternatively, we will evaluate the probe concentration at the signal site by ICP-MS. If assessment of targets on AuFe probes by ICP-MS is not conclusive, alternatively we will use Analytical ultracentrifugation (AUC), to quantify the proteins on nanoparticles (separation based on molecular weight).
Specific Aim 2. Develop a multiplex mLF-IC platform for simultaneous detection of two protein targets and evaluate the performance metrics.
In Aim 2, we will evaluate the detection parameters (probe concentration and capture efficiency) for multiplexing in purified and in cervical cancer tissue swab samples.
A) Multiplex detection. Printing of capture ligands (antibodies and aptamers) is a good process to accomplish multiplex detection. In this application, we will extend the technology to detect two targets (VCP and p16INK4a) along with HPV. Patterning enables precise positioning of the antibodies at the test zone. We will use established procedures to pattern the antibodies on the mLF-IC strip (Fig. 3). Experiments will be selectively conducted to assess flow fields and binding ratio for the following conditions at the capture sites for VCP and p16INK4a (‘X’ implies no antibodies are printed at the test zone for the biomarker): (X,X), (VCP,X), (X, p16INK4a), (VCP, p16INK4a) along with HPV. We will utilize experimental conditions for concentrations (25 fg/ml – 500 ng/ml) with purified proteins for multiplex detection. Complementary analysis with ICP-MS will provide information on NP probe concentration at the respective capture site. With information on the NP concentration, results from AUC, the average concentration of targets per particle can be obtained. Knowing the initial concentration from Mass spectroscopy, the capture ratio at the signal generation site can be estimated.
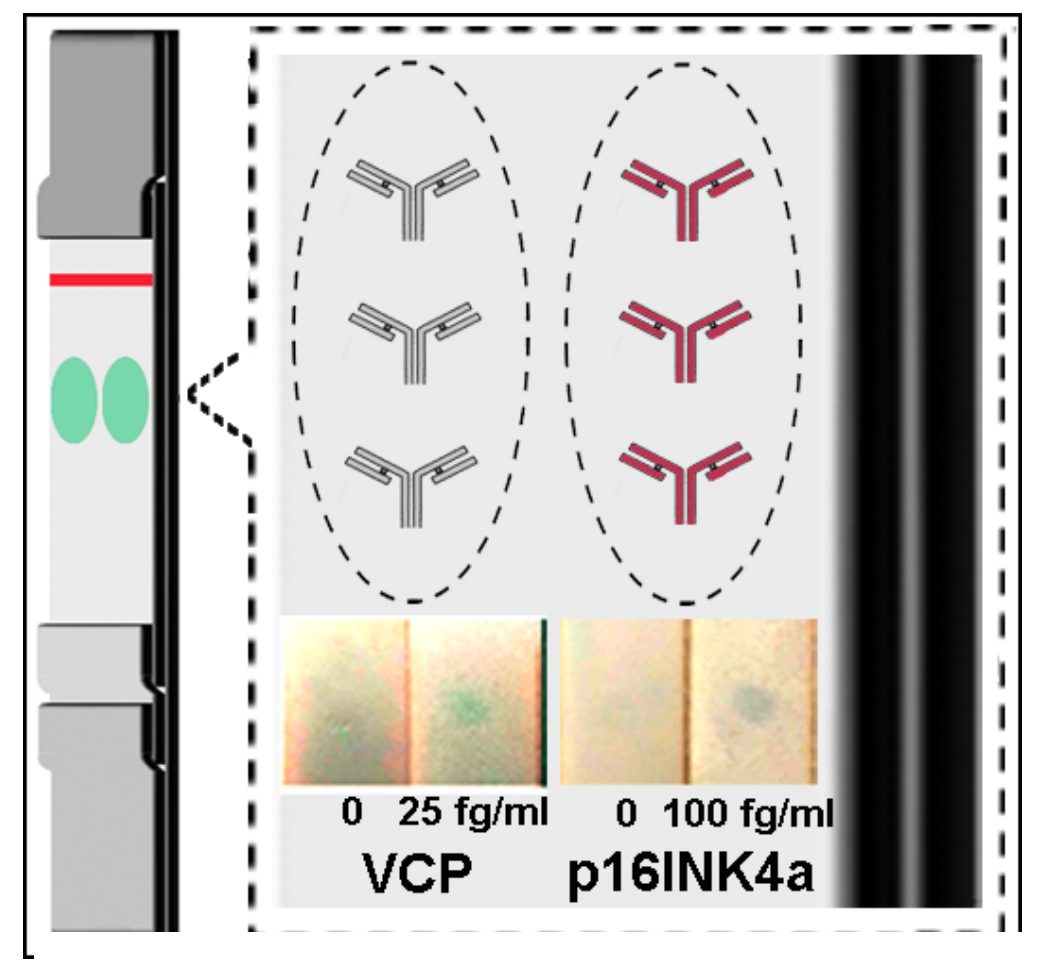
Fig. 3. Multiplex detection of VCP & p16INK4a
B) Sample Preparation: Tissue swabs will be collected from women undergoing screening for cervical cancer at the Vinmec International Hospital in Vietnam. Cytology reports is available will accompany these samples. In 2022, at Vinmec there were 23,921 cytology-based screening tests for cervical cancers, out of which 301 cases were detected as cancer/pre-cancer (including ASC-H, LSIL, and HSIL) and had to be followed up with biopsy diagnostics. We have prior experience in handling tissue samples, wherein we obtained 50 samples from consented patients under a full IRB approval (Category 4 exempt IRB #1605017751 through a collaborative work with Dr. Mohammed from Purdue University). Samples were stored and shipped in RNALater stabilization solution to minimize the need to immediately process tissue samples or to freeze samples in liquid nitrogen for later processing. The samples could then be centrifuged to obtain cell pellets which can be lysed using FastBreak cell lysis buffer (Promega), used for efficient and gentle lysis of cell culture without the need for centrifugation or mechanical disruption. Following a brief incubation, the cells can be disrupted, and the protein released for experiments. Our main goal is the assessment of samples to establish performance metrics. We do not foresee any hurdles in this step because of our prior experience. As an alternative we will employ aptamers targeting the proteins to assess sensitivity and specificity.
C) Deliverables. Upon completion of Aim 2, we will be able to assess the LOD and LOQ to detect the two protein biomarkers simultaneously in relation to HPV. Evaluation tests will be run concurrently at VinUniversity Biolab and the nearby Vinmec International Hospital in Vietnam. Our goal is to evaluate on 1000 samples that go through regular cervical cancer screening at Vinmec.
D) Pitfalls and Alternatives. Considerations discussed above apply. The extent of noise due to cross-reactivity of antibodies/antigens will be assessed. Since we can detect nucleic acid targets, alternatively, we will conduct select nucleic acid target detection for markers listed in Table 1 for cross validation.
Quantitative Milestones
Specific Aim 1: Establish the limit of detection (LOD) and the limit of quantification (LOQ) in in vitro samples.
Milestone 1: Quantify the target capture ratio at the signal generation site for various initial concentrations (25 fg/ml – 500 ng/ml) of the target – quantify the number of targets per probe and capture ratio at the signal zone.
Milestone 2: Develop image processing algorithms on smartphone apps (both in Android and iOS) to automatically detect the signal generation sites on the LF strip and quantify the colorimetry of these sites. Captured LF images and extracted results are uploaded from the smartphone to a cloud for later reviewing and analysis.
Milestone 3: Achieve LOD of 25-100 fg/ml and LOQ in the range 25 fg/ml-100 ng/ml and construct calibration curves.
Specific Aim 2: Evaluate LOD/LOQ for the two protein markers along with HPV in purified and tissue swab samples.Milestone 4: Achieve an LOD for the 2 biomarkers (purified proteins) and (LOQ) to quantify 25 fg/ml – 25 ng/ml in separate samples and in mixed samples in the following ratio (1:0, 1:0, 0.5:0.5, 0.25:0.75, 0.75:0.25). Five biological replicates will be used. Achieve standard error/variance of <15%.
Milestone 5: Establish LOD and LOQ levels to evaluate protein levels in tissue samples. Use 1000 samples that go through regular cervical cancer screening at Vinmec International Hospital. The mLF-IC performance will be also compared to the HPV and cytology-based results. The comparison will be reported as Cohen’s kappa, positive percent agreement (PPA), negative percent agreement (NPA). The two-sided 95% score confidence intervals for PPA and NPA will be also provided.
Innovation and Transformative Potential. Our approach represents a novel yet a practical means to redefine the sensitivity and LOD of LF colorimetric assays. Our goal is to make a profound effect on POC diagnostics not just for cervical cancer, but also for other diseases with a focus on low resource environments with students embedded in this research from inception with advisors from VinUni and Illinois. The global demand for LF-based tests will continue to rise, future efforts in this market space need to address sensitivity, reproducibility, quantification, multiplexing, and cross-platform detection capability utilizing intelligent analysis algorithms [16]. It is expected that our work will have the potential to transform lateral flow assays to near single molecule sensitivity and with ability to quantify targets. Further, once the methods are established to evaluate the design and performance for protein-based targets, we will extend to quantify transcripts, as in HPV, and compare with commercial systems. In addition to defining the detection limits and performance metrics of the technology utilizing purified proteins, we will validate the technology with tissue samples. In the long-term these methods can be extended for cervical cancer staging based on the clinical sensitivity and specificity. Partnership with a commercial vendor is a possibility after clinical trials.
Team Members[1] : To accomplish the tasks, we have established a multidisciplinary team comprising of engineers and cancer biologists, and clinicians with expertise in pathology, microbiology, cancer biology, image analysis including machine learning, biosensors with specific expertise in lateral flow technology development. The members are:
Prof. Joseph Irudayaraj (PI, Dept. Bioengineering, UIUC) [https://scholar.google.com/citations?user=TVIMFxkAAAAJ]
Prof. Minh Do (Department of Electrical and Computer Engineering, UIUC) [https://orcid.org/0000-0001-5132-4986] Prof. Tung Nguyen (College of Engineering and Computer Science, VinUni) [https://orcid.org/0000-0003-0275-6517] Dr. Tu Phan (VinUni-Illinois Smart Health Center, VinUni) [https://orcid.org/0000-0002-6590-046X]
Dr. Phuong Doan (Health Sciences, VinUni; Microbiology, Vinmec) [https://vinuni.edu.vn/people/doan-mai-phuong-md-phd/] Dr. Hang Nguyen (Health Sciences, VinUni; Pathology, Vinmec) [https://vinuni.edu.vn/people/nguyen-thi-hang-md/]
Expected Outcomes:
(i) External Funding: Beyond VinUni-Illinois Smart Health Center funding, after obtaining promising clinical validation we will submit proposals to external funding sources including USAID, Bill & Melinda Gates foundation, and Wellcome foundation, to address low-cost early screening for other types of cancers in LMICs.
(ii)Publications: Given that we will have a significant number of patient samples and the project involves evaluation of both protein and nucleic acid biomarkers, we expect to publish 2-3 collaborative publications/year. In addition, the students will have the opportunity to collaborate in other cancer related projects while at UIUC to broaden their research experience.
(iii) Patents: As technology development evolves, we will file for provisional patents per the agreement between the institutions.
(iv) Technology Transfer Activities: Once optimized and tested at UIUC, the technology will be transferred to VinUniversity and prototypes will be replicated in their Biolab for clinical studies.
(iv) Clinical Studies: Patients will be recruited at the Vinmec International Hospital in Vietnam and test strips fabricated at VinUniversity will be used for testing with the high resolution mLF-IC and correlated with the standard of care used at present.
Request: We request two PhD students to work on the proposed research under the joint supervision of faculty team members at UIUC, VinUni, and Vinmec. One PhD student will work on technology development and the other PhD student will work on sample preparation, biological evaluation and clinical testing. We will have biweekly meetings via zoom and expect quarterly progress reports. The students will attend atleast one conference during their time in Illinois.
Timetable:
| Timetable of Milestones (Year) | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | |
| 1. | Optimization of reagents, determine capture efficiency at the signal generation site using in vitro samples, development of machine learning algorithms. | x | x | x | x | ||
| 2. | Establish LOD, calibration standards for cervical cancer biomarkers | x | x | x | |||
| 3. | Develop and evaluate image processing algorithms for LF colorimetry on smartphone | x | x | x | x | ||
| 4. | Evaluate performance metrics using protein and nucleic acid capture agents | x | x | x | x | ||
| 5. | Validate technology & assess performance metrics in tissue swab samples. | x | x | x | |||
References
- Tran, K., Park, Y., Kim, B., Oh, J. and Ki, M. Incidence and mortality of cervical cancer in Vietman and Korea (1999-2017). Epidemiology Health. 42:e2020075 (2020).
- Sung, H., Ferlay, J. et al., and Soerjomataram, I. Global Cancer Statistics 2020: GLOBOSCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. May 2021: pp.209-249 (http://globocan.iarc.fr/ and http://www.who.int/)
- Sharmila, P. and Mishra, G. Cancer cervix: Epidemiology and disease burden. Cytojournal. 19:21 (2022).
- Denny, L., Cervical cancer prevention: New opportunities for primary and secondary prevention in the 21st century.Int. J. Gynecology & Obstetrics, 2012. 119(S1).
- Wright Jr, T.C. and L. Kuhn, Alternative approaches to cervical cancer screening for developing countries. Best Practice & Res. Clin. Obstet. & Gyn, 2012. 26(2):197.
- Louie, K.S., S. De Sanjose, and P. Mayaud, Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: a comprehensive review. Tropical Medicine and International Health, 2009. 14(10):1287-1302.
- Unger, E.R., et al., Molecular markers for early detection of cervical neoplasia. Disease Markers, 2004. 20(2): 103.
- Gaffikin, L., M. Lauterbach, and P.D. Blumenthal, Performance of visual inspection with acetic acid for cervical cancer screening: a qualitative summary of evidence to date. Obstetrical and Gynecological Survey, 2003. 58(8):543-550.
- Sankaranarayanan, R., et al., Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. The Lancet, 2007. 370(9585):398-406.
- Schiffman, M., et al., Human papillomavirus testing in the prevention of cervical cancer. Journal of the National Cancer Institute, 2011. 103(5):368-383.
- Ratnam, S., et al., Aptima HPV E6/E7 mRNA test is as sensitive as hybrid capture 2 assay but more specific at detecting cervical precancer and cancer. Journal of Clinical Microbiology, 2011. 49(2):557-564.
- Mohammed, S.I., et al., Point-of-care test for cervical cancer in LMICs. Oncotarget, 2016. 7(14): p. 18787.
13.Ren, W., Mohammed, S., and Irudayaraj, J. Magnetic focus lateral flow sensor for detection of cervical cancer biomarkers. Anal. Chem., 91 (4): 2876-2884 (2019).
- Ren, W., Ahmad, S., and Irudayaraj, J. 16S rRNA monitoring point-of-care magnetic focus lateral flow sensor. ACS Omega, 6 (16):11095-11102 (2021).
- Ren, W., et al., Ultrasensitive detection of microbial cells using magnetic focus enhanced lateral flow sensors.Chemical Comm., 2016. 52(27):4930-4933.
- Budd, J., et al., Lateral flow test engineering and lessons learned from COVID-19. Nature Reviews Bioengineering, 2023. 1(1):13-31.
17. Rivas, L. Improving sensitivity of gold nanoparticle-based lateral flow assays by using wax-printed pillars and fluidics. Lab on a Chip, 14(22):4406-14 (2014).
- Tsujimoto, Y., et al., Elevated expression of valosin-containing protein (p97) is associated with poor prognosis of prostate cancer. Clinical Cancer Research, 2004. 10(9):3007-3012.
- Yamamoto, S., et al., Expression level of valosin-containing protein (p97) is associated with prognosis of esophageal carcinoma. Clin. Cancer Res., 10(16): 5558 (2004).
- Julien, S.G., et al., Inside the human cancer tyrosine phosphatome. Nat Rev Cancer, 2011. 11(1):35-49.
- Serrano, M., G.J. Hannon, and D. Beach, A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature, 1993. 366(6456):704-707.
- Jing, M., et al., Degradation of tyrosine phosphatase PTPN3 (PTPH1) by association with oncogenic human papillomavirus E6 proteins. J. Virology, 2007. 81(5):2231.
- Schmidt, D., et al., p16/ki‐67 dual‐stain cytology in the triage of ASCUS and LSIL Papanicolaou cytology. Cancer cytopathology, 2011. 119(3):158-166.
