Point of Care and Telehealth Diagnostics for Data-Driven Smart Health Systems
Abstract
Cloud-based telehealth services are rapidly becoming an integral aspect of inexpensively providing broadly accessible, convenient, and effective access to healthcare. Driven by the nearly universal adoption of smartphones, combined with a broadening of the medical services provided by neighbourhood clinics and pharmacies, people are becoming empowered to actively participate in maintaining the health and wellness of themselves and their families while leading busy lives that are significantly disrupted by lengthy visits to traditional medical facilities. Importantly, high-quality diagnostic health data is a fundamental requirement for physicians to provide accurate early disease diagnosis, to precisely select the most effective course of therapy, and to monitor wellness status that supports lifestyle choices to prevent chronic disease. Due to the cost and complexity of conventional laboratory-based diagnostic tests, the current paradigm for detection of pathogen infection, cancer diagnosis, cardiac health monitoring, maternal health monitoring, and measuring the effects of diet, exercise, and anxiety are expensive and invasive, leading to infrequent usage and a sparse data record over the lifespan. There is a pressing need to develop a new generation of diagnostic technologies that are compatible with the combined characteristics of low-cost instrumentation, simple assay methods, high sensitivity, accurate quantitation, and rigorous validity. We envision the creation of diagnostic technologies that can be deployed in home-based self-testing scenarios that are linked to a patient’s mobile device, as well as point of care scenarios that are used in clinics. When utilized as data-collection devices within a broader cloud-based telehealth medical service system, such devices will provide key information to physicians that will enable longitudinal monitoring, and personalized data-driven decision-making to complement healthcare delivered by conventional hospitals and medical centers. Our proposed project will develop and apply novel physics and molecular biology principles in three areas to achieve these goals. First, photonic metamaterials will be designed, fabricated, and utilized to substantially amplify the interactions between light and biological analytes to enable digital-resolution detection of viral pathogens, genomic biomarkers, and protein biomarkers. Second, micro-electro-mechanical sensors (MEMS) will be developed as miniaturized, high-sensitivity devices fabricated using high-scalable manufacturing methods to serve as multi-modality multiplexed sensor arrays within handheld, smartphone-linked self-testing instruments. Third, we will utilize nucleic acid engineering to devise novel, ultraselective, rapid assay methods that operate in a single step at room temperature without enzymatic amplification or washing steps. Our project will pave the way toward a substantially more robust and high quality collection of biomarker data that, when integrated with a telehealth service system, will form the basis of mass-market products and services for health management.
Background and Significance
Although laboratory-based molecular biomarker diagnostic tests have become a broadly adopted aspect of medical care, the most widely adopted assay methods, such as Enzyme Linked Immunosorbent Assay (ELISA) and Polymerase Chain Reaction (PCR) utilize complex workflows that require enzymatic reactions to achieve high sensitivity, precise temperature control, washing steps, protein-based reagents, and expensive detection systems that make them well-suited only for highly-trained technicians working in a laboratory environment. While laboratory automation effectively reduces hands-on time, errors, and cost/test, only the simplest tests (such as lateral flow test strips and enzyme-based electrochemical tests) have successfully made the transition to self-test environments. A conventional strategy for lab-on-a-chip, point of care testing is to miniaturize a conventional laboratory method into a microfluidic cartridge, sometimes combined with slightly modified molecular biology methods that provide less stringent requirements for thermal control, or an alternative readout method in which a conventional assay is performed in combination with a biosensing transducer. Further alternative strategies move toward “label-free” detection of molecular biomarkers or viral pathogens, but typically at the cost of poor sensitivity compared to label-based alternatives.
Recently, the Cunningham group described three novel forms of microscopy that utilize photonic crystal (PC) optical metamaterial surfaces to efficiently couple energy from external light sources (LEDs and lasers) into surface-confined resonant optical modes that substantially enhance scattering, absorption, and fluorescence emission from bio-nano-materials (nanoparticles, biomolecules, viruses, and exosomes) that come into contact with the PC surface by either diffusion or by selective probe capture [1-6]. Utilizing gold nanoparticles, quantum dots (QDs), and plasmo fluors as tags for biomolecular assays, we are demonstrating the ability to digitally count individual biomarker molecules (miRNA, cfDNA, proteins) from a single-droplet clinical sample, with detection limits down to 100 aM, while maintaining quantitation over >5 logs concentration range. Importantly, the new microscopies, called Photonic Resonator Absorption Microscopy (PRAM) and Photonic Crystal Enhanced Fluorescence (PCEF) enable the assays to be performed with low-power LED or laser diode illumination and inexpensive webcam-quality image sensors.
An important aspect of our project derives from recently reported advances in the Wang group, in the application of customized “DNA origami” structures with sophisticated designs that form the shape of nets, whose vertices match the pattern of outer proteins expressed on the surfaces of Dengue, SARS-CoV-2 and HIV-1 viruses [7, 8]. The Designer DNA Nanostructures (DDNs) are effectively a reagent that, mixed with a virus-containing clinical sample, enables the release of fluorophores that are easily detected with a palm-sized, smartphone-linked fluorimeter called the “V-Pod” (because it resembles an Apple AirPod™ headphone case). DDN-based detection with the V-Pod fluorimeter provides a single-step, 10-minute self-test assay that links to a smartphone-based contact tracing service. Additionally, using thermodynamic principles that guide the self-assembly of highly functional nucleic acid structures from inexpensive raw materials (synthetic DNA sequences), structures comprising DNA “toehold probes” linked to gold nanoparticles or QDs serve as highly selective probes for miRNA biomarkers, used in conjunction with PRAM and PCEF detection to provide room temperature, enzyme-free, single-step, ~1-15 minute assays.
Dr. Quynh is establishing a research group at VinUniversity to investigate the reduced dimension of optical metamaterials, called metasurfaces, for biosensing purposes. The metasurface-based biosensors have attracted significant attention due to the excellent detection limits afforded by enhanced light-matter interactions. He will harvest his previous momentum during his PhD and postdoc training to further develop metasurface-based biosensors. His experience in developing plasmonic metasurfaces for various applications [9] is strongly coupled to the goals of this project. Importantly, he demonstrated near-infrared metasurfaces to enhance the photoluminescence of QDs and the electroluminescence of QLEDs. He also worked with suspended semiconductor membranes to achieve the lasing from bound states in the continuum (BIC)– a remarkable state of the structures in which the light is totally confined. The exceptional high Q-factors of the BICs would make the dielectric metamaterials become an ideal candidate to push biosensor detection limits. In addition to the exotic physical properties that are promises for biosensing, advances in fabrication allow us to produce dielectric metasurfaces in manufacturable volumes using silicon microfabrication methods to achieve low costs that are compatible with point-of-care diagnostic applications.
The main emphasis for the project will be the research and development of self-testing and point-of-care testing technologies that can be broadly applied in many health/wellness contexts. Therefore, we will demonstrate the system’s capabilities using two representative classes of biomarkers: 1. Micro RNA (miRNA) and 2. Proteins.
Recently microRNA (miRNA) has emerged as a promising clinical marker in cancer, cardiac disease, nutrition, and chronic stress [10, 11]. These ~21-25 nucleotide non-coding RNAs broadly regulate gene expression and exhibit significantly altered levels in states of disease, with a high correlation with disease state. Importantly, miRNA is abundant in exosomes, which are ~30-100 nm extracellular vesicles (EV) secreted by cells that contain molecular profiles of the cell from which they were derived [12, 13]. Exosomes are found in all bodily fluids, where they protect their nucleic acid contents from degradation [14]. Thus, exosome-derived miRNAs are a highly promising and readily accessible reservoir of information on the dynamic changes that cells undergo during cancer progression and during the response to therapy.
As a representative protein, we will focus upon the detection of Troponin I and T, which are specific and sensitive biomarkers of myocardial injury. They are the recommended serologic tests for individuals suspected of having an acute myocardial infarction (MI). Accurate and timely identification of acute (thrombotic) MI is critical, since successful care requires the use of specialized antithrombotic medicines and, in many cases, percutaneous coronary intervention. Troponin is the preferred blood-based test in the diagnostic evaluation of patients suspected of acute MI, and an elevated value is required in most cases. Self-testing of cardiac troponin I and T can potentially save lives, as Troponin levels in the heart often begin to increase two to three hours following the onset of acute MI.
Goals
Extending from the recent research accomplishments of the investigators, our project aims to assemble a multidisciplinary collaboration with the goal of developing, demonstrating, and characterizing point of care and self-testing diagnostic technologies that take advantage of the unique properties of photonic metamaterials, MEMS sensors, and molecular biology methods using engineered nucleic acid probes. Our goals are summarized:
1. Utilizing the V-Pod fluorimeter as an initial concept of a self-test instrument, design and construct a family of inexpensive, palm-sized, smartphone-linked “Pod” instruments that utilize MEMS sensors, fluorescence emission, and colorimetric absorption as the detection modality for single-step diagnostic tests performed in a single 0.2 mL PCR tube. Develop a “Tri-Corder” that integrates all three detection modalities into a single Pod. A smartphone app controls all instrument functions, gathers data, and reports data to a cloud-based record.
2. Using the PRAM and PCEF detection instruments that utilize TiO2 and Si3N4 PC surfaces as a basis for digital resolution molecular biomarker detection, design, construct, and test point-of-care versions of the instrument that utilize conventional CMOS image sensors and LED illumination. Design, fabricate, and test novel photonic metamaterial structures to further enhance light-matter interactions for enhanced sensitivity.
3. Utilizing engineered nucleic acid probes, develop simple single-step assay workflows for miRNA and protein (Troponic) biomarkers from blood plasma that utilize the detection instruments in Aims 1-2.
4. Validating the performance of combined assays with detection instruments in collaboration with VinMec clinicians. The process will follow the requirements of the hospital with IRB approval.
Approach
We plan to develop a two-tiered POC platform (Fig. 1) that can either interface wirelessly with a smartphone, or be used for more complex tests in clinical settings. The platform will use various methodologies: optical metamaterials, microfabricated electrochemical biosensors, or assays that generate a coloured/fluorescent liquid change in response to the presence of target analytes. AI algorithms on the smartphone help to analyze, classify, early-diagnostic and detect abnormalities from the sample.
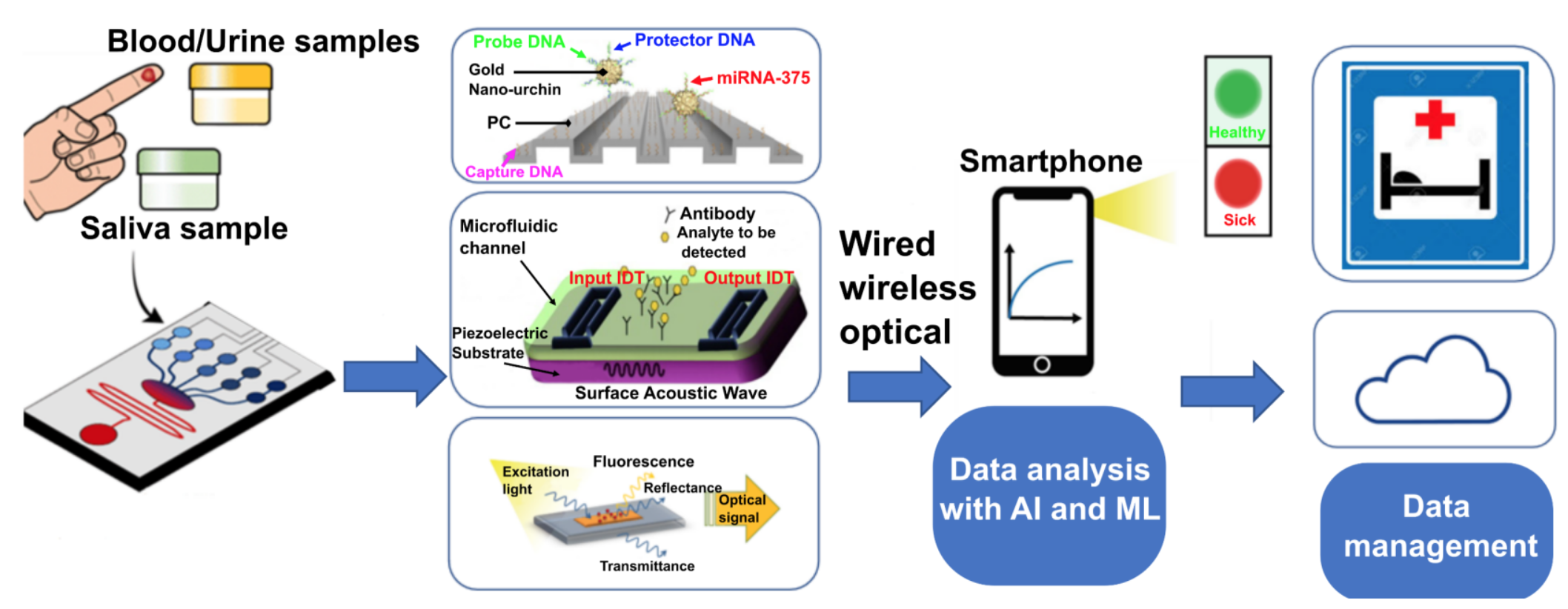
Figure 1. Schematic overview of the self-testing and point of care detection systems
Developing future talents is one of the key objectives of the project. We will work closely with the Vingroup scholarship team for advertising, interviewing, and recruiting graduate students with matching backgrounds. Based on the nature of the multi-disciplinary fields, and the time-line requirements at each stage, our plan to recruit and co-advise PhD, MS students, and postdoctoral scholars:
1st year: Recruit 1 PhD and 1 MS student with a biomedical/bioelectronics/physics background. The PhD student will enrol in UIUC. The Master student will enrol in VinUni (as VinUni does not yet offer a PhD in engineering); Recruit 1 postdoc in chemistry or life science co-advised by VinUni and UIUC faculty.
2nd year: Recruit 1 PhD student in chemistry or life science enrolling in UIUC.
3rd year: Recruit 1 PhD student in EECS enrolling in VinUni. 1 MS student will enrol in VinUni.
4th year: Recruit 1 PhD student with a biomedical, bioelectronics background enrolling in VinUni (Anticipating VinUni will have an engineering PhD program at that time).
5th year: Recruit 1 PhD student in EECS enrolling in VinUni. 1 MS student will enrol in UIUC.
Referenced Literature
1. Canady, T.D., et al., Digital-resolution detection of microRNA with single-base selectivity by photonic resonator absorption microscopy. Proc Natl Acad Sci U S A, 2019. 116(39): p. 19362-19367.
2. Che, C., et al., Activate capture and digital counting (AC + DC) assay for protein biomarker detection integrated with a self-powered microfluidic cartridge. Lab Chip, 2019. 19(23): p. 3943-3953.
3. Huang, Q., et al., Enhanced plasmonic photocatalysis through cooperative plasmonic-photonic hybridization. ACS Photonics, 2020. 7(8): p. 1994-2001.
4. Huang, Q., et al., Critical Review: digital resolution biomolecular sensing for diagnostics and life science research.Lab Chip, 2020. 20(16): p. 2816-2840.
5. Li, N., et al., Photonic Resonator Interferometric Scattering Microscopy. Nature Communications, 2021. 12: p. 1744.
6. Xiong, Y., et al., Photonic crystal enhanced excitation, directional extraction, and blinking suppression for single quantum dot digital resolution biosensing. Nature Communications, 2021. In revision.
7. Kwon, P.S., et al., Designer DNA architecture offers precise and multivalent spatial pattern-recognition for viral sensing and inhibition. Nat Chem, 2020. 12(1): p. 26-35.
8. Chauhan, N., et al., Programming designer DNA nanostructures for SARS-CoV-2 rapid detection and potent inhibition. In revision, 2021.
9. Le-Van, Q., et al., Electrically driven optical metamaterials. Nat Commun, 2016. 7: p. 12017.
10. Peng, Y. and C.M. Croce, The role of MicroRNAs in human cancer. Signal Transduction And Targeted Therapy, 2016. 1: p. 15004.
11.Hayes, J., P.P. Peruzzi, and S. Lawler, MicroRNAs in cancer: biomarkers, functions and therapy. Trends in Molecular Medicine, 2014. 20(8): p. 460-469.
12. Vlassov, A.V., et al., Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta, 2012. 1820(7): p. 940-8.
13. Gyorgy, B., et al., Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci, 2011. 68(16): p. 2667-88.
14. Boukouris, S. and S. Mathivanan, Exosomes in bodily fluids are a highly stable resource of disease biomarkers.Proteomics Clin Appl, 2015. 9(3-4): p. 358-67.
