Evaluating the Effect of Antiviral Drugs using Polarized Light Imaging and Machine Learning Approaches: The Case of Human-induced Pluripotent Stem Cell-derived Cardiomyocytes
Abstract
Frequently the same disease treatment can have different effects in different people. Hence, finding the safest and most effective treatment for an individual is critical in today’s personalized medicine. If drug evaluation could be performed safely ex vivo at the cells/tissue level using imaging techniques, a significant advance would occur. A standard technique for ex vivo assessment is based on using fluorescence dyes and labels, however, they perturb the cellular environment and interfere with cellular health and function.
We propose to develop a standard and robust procedure to evaluate the effectiveness of antiviral drugs using label-free, noninvasive light imaging and machine learning-based approaches. To demonstrate this with a representative example, we will start with the evaluation of the effects of Molnupiravir, used for treating SAR-CoV- 2, on cardiomyocytes derived from a human-induced pluripotent stem cell. This project will then be extended tothe evaluation of other antiviral drugs. The outcome of this project will not only answer fundamental questions about cell dynamics corresponding to the treatment, but also have a translational aspect and high impact at the societal level if routinely adopted and used in clinical settings.
Significance
- Establishing a non-invasive and label-free imaging technique for cell imaging. The polarized light imaging technique developed at VinUni is a simple and cost-effective method that can be adapted to any current bright field microscope.
- This will be the first time that polarized images of cardiomyocytes are recorded in our institution, and these will be used to build libraries with different image data corresponding to various diseases in order to effectively and safely establish their treatments.
- This would also be the first time AI tools will be used to evaluate the medication effectiveness based on 2-D polarized and other nonlinear label-free images. We also aim to compare the performance of AI algorithms trained on 2-D polarized imaging against other imaging modalities.
- As a unique goal of this grant, this project will help to train Ph.D. and M.S. students with different science backgrounds. In addition, this project will provide an excellent opportunity for VinUni students to spend time at the University of Illinois Urbana-Champaign where they will learn to use many different advanced label-free, noninvasive optical imaging techniques and apply them for further studies at VinUni.
Goals
- Aim 1: Build hardware (Polarized Light Microscope) and develop experimental procedure to image a single cell in vitro. (VinUni)
- Aim 2: Evaluate the cardiotoxicity of the short-term and long-term treatment of antiviral drugs in human-induced pluripotent stem cell-derived cardiomyocytes from healthy individuals and patients. Through this project, we will accumulate an extensive dataset and build a lookup library of cytotoxicity for each antiviral drug at the cell level. (VinUni)
- Aim 3: At the University of Illinois, VinUni students will be trained to use advanced nonlinear, label-free optical systems for imaging cells and tissues at the cellular and sub-cellular levels. This expertise will be transferred to VinUni for further studies. (UIUC)
- Aim 4: With the most recent AI developments for healthcare applications, deep learning will be used to identify the cytotoxicity of the drugs based on the myofibril length, myofibril density, retardance, and orientation of the sarcomeres. (VinUni and UIUC)
Approach
Aim 1: Hardware and experimental procedures for polarized light imaging of treating cells (Dr. Mai Tran)
Polarized Light Imaging is a powerful tool for investigating the structure and dynamics of living cells and tissues [1, 2]. It is a non-invasive imaging technique that can measure the retardance and slow axis orientation at a single-pixel resolution. However, to our knowledge, there is no study on using polarized light imaging to evaluate cell treatment. LC-PolScope is one type of Polarized Light Microscope that replaces two crossed analyzers with a universal polarizer and circular analyzer (Fig. 1). This imaging technique can determine the optical polarization parameters in many specimen points simultaneously, in short time intervals, and at the highest resolution without mechanical changes to polarization components in the optical path. A detailed description of the LC-PolScope was described elsewhere [3]. Briefly, as shown in Fig. 1(A), the universal polarizer generates any polarization setting. In our study, we will use five polarization settings of incoming light. While acquiring the five raw images in Fig. 1A, no part of the optical setup was moved or rotated, including the specimen. The only adjustment was the voltages applied to the liquid crystals. The intensity of a given image point in the five raw images is called I0, I1, I2, I3, and I4. The retardance, the azimuth, and the swing are R, φ, and χ, respectively. By measuring these parameters, we can observe the structure and dynamics of the live cells and tissues.
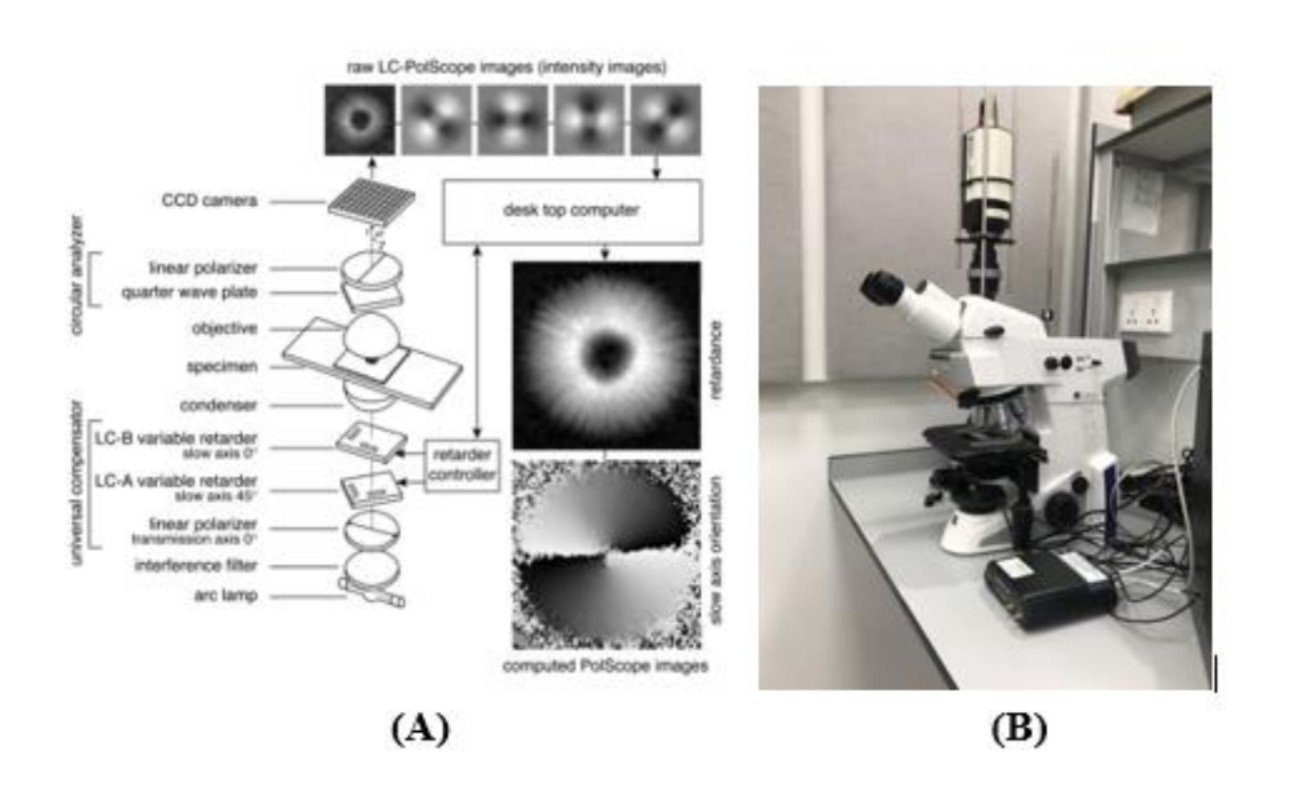
Figure 1. (A) Schematic of the LC–PolScope. In the top row are five raw images of isolated aster from surf clam eggs corresponding to five different settings of the universal compensator. The computer produces retardance and slow-axis images based on the raw images. Reprinted from [3]. (B): The LC-PolScope at Prof. Tran’s lab.
This microscope is available at Prof. Tran’s lab (Fig. 1B) and will be adapted and optimized for cell treatment evaluation. We propose the streamlined procedure as follows:
Step 1: Cells are imaged with the bright field microscope and the LC-PolScope to serve as a reference.
Step 2: Cells are again imaged with the bright field microscope and the LC- OpenPolScope after addition of medication at different time points based on the suggestion of clinicians.
Step 3: Images obtained at different times will be compared to see any changes in the structures or dynamics of the treated cells. Treated cells will also be compared with the untreated cells and cells from healthy subjects to establish the length and frequency of treatment with medication that gives the best effectiveness.
Aim 2: Evaluation of the effects of Molnupiravir on the cardiomyocytes derived from human induced pluripotent stem cells (Dr. Mai Tran, Dr. Nhung Nguyen)
As the first biological/biomedical question, we chose to explore how cardiovascular cells are changed under Molnupiravir treatment. The coronavirus COVID-19 pandemic is a global health crisis, and the updated data shows that over 500 million people got the infection. In addition, post-Covid complications have become more common, mainly triggered by the SAR-CoV- 2 viral infection and possibly by the medication treatment. Molnupiravir is an antiviral drug granted emergency use authorization by the U.S. FDA. Drug safety and the effect of Molnupiravir are still under intensive investigation. However, literature has documented a high incidence of cardiovascular dysfunction to antiviral treatment, including left ventricular hypertrophy, diastolic dysfunction, and hypertension [4-6]. Several studies also report that Remdesivir, another widely used antiviral drug, significantly causes cardiotoxicity [7, 8]. This accumulated evidence suggests that these antiviral drugs can potentially exert serious adverse effects on the cardiovascular system. Therefore, discovering the impact of drugs on the cardiovascular system is vital for establishing their cytotoxicity and safe consumption. Commonly, researchers use fluorescence imaging techniques to observe cellular morphology and physiological functions. As shown in Fig. 2, each embryonic stem cell–derived cardiomyocyte (ESC-CM cell) was analyzed for average sarcomere length, cell area, cell perimeter, and cell circularity index. Images obtained from early-stage hESC-CMs suggest a highly underdeveloped contractile machinery composed of low-quality misaligned myofibrils (Fig. 2A, B). Spanning between these structures were thick filaments composed of myosin at a relatively low density. In contrast, late-stage hESC-CMs showed significant improvements in myofibril alignment, density, and morphology (Fig. 2C, D). In that report, the authors used optical video microscopy to track developmental changes in the contractile properties of hESC-CMs and hiPSC-CMs. Early-stage PSC-CMs lack visible sarcomeres, so we quantified contractile performance by monitoring changes in the cell length. In late-stage hESC-CMs, it is better to measure the contractile performance to monitor sarcomere length changes during contraction.
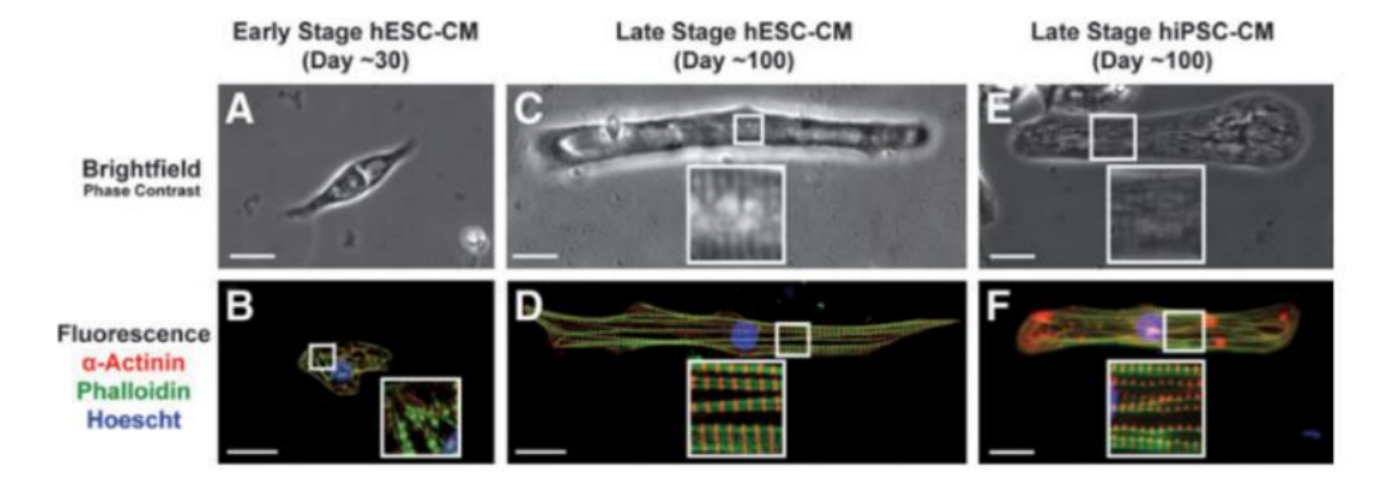
Figure 2. Microscopy images of ESC-CM cell at different stages of development obtained with phase-contrast microscopy (A-C-E) and fluorescence microscopy (B-D-F). Scale bar 25 m. Adapted from [9].
Inspired by this work, we propose using the LC-PolScope for the evaluation of the effects of Molnupiravir on cardiomyocytes derived from human induced pluripotent stem cells. With growth, cells show dramatic differences in morphology, including increased cell size and anisotropy, greater myofibril density and alignment, and sarcomeres visibility. Additionally, the architectural dynamics of the cellular components using polarized fluorescence microscopy are also revealed. As a result, in this study, we want to use the LC-PolScope to test whether Molnupiravir directly induces harmful effects on cardiomyocytes regarding cellular morphology and physiological processes. We propose research to examine both short-term and long-term treatment of Molnupiravir in human-induced pluripotent stem cell-derived cardiomyocytes. Label-free, noninvasive optical imaging can potentially be applied for different antiviral drugs and disease models as a powerful tool to evaluate the safe and effective drug treatment in cardiomyocytes or heart tissue.
Aim 3: Using advanced nonlinear, label-free optical systems for imaging cells and tissues at the cellular and sub-cellular levels (Prof. Boppart, Prof. Marjanovic)
In the Biophotonics Imaging Laboratory (BIL) at UIUC, there are a variety of advanced microscopy techniques developed to visualize and characterize cells and tissues for different biomedical applications. These include optical coherence tomography (OCT) [10-13], multiphoton microscopy [14-17], and nonlinear Raman spectroscopy [18, 19]. Students from VinUni will be trained on the working principles and the designs of these imaging systems and get hands-on experience in both imaging and data analysis. They will be able to adapt this knowledge to develop their own advanced imaging systems at VinUni in the future for their specific applications. In particular, students can learn advanced polarization sensitive imaging techniques to complement the polarized light microscope system at VinUni. In BIL, we also developed a co-registered polarization-sensitive optical coherence tomography and microscopy (PS-OCM) and polarization-sensitive SHG (P-SHG) system to better characterize collagen fibers in fresh tissue (Fig. 3) [20, 21].
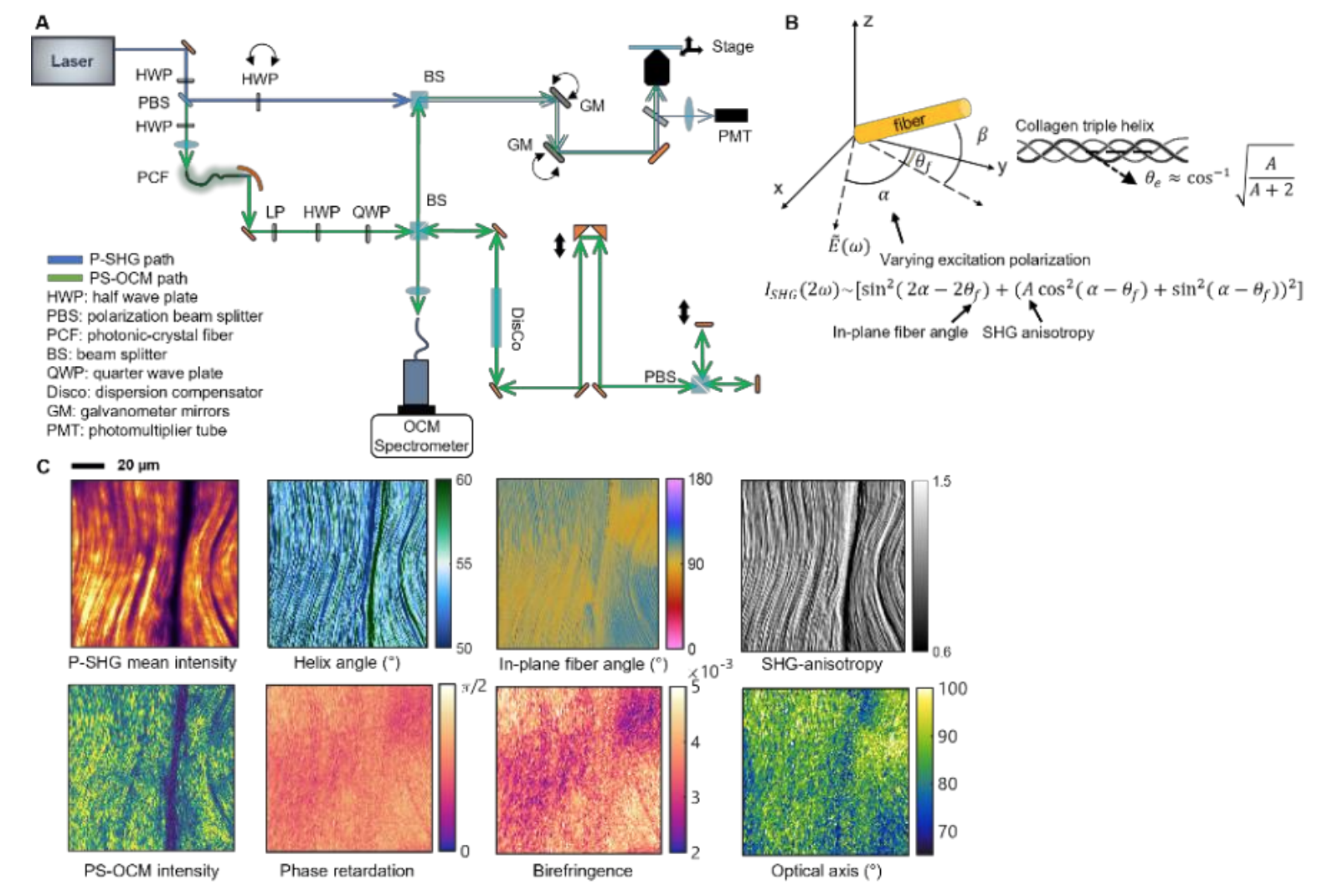
Figure 3. Co-registered and simultaneous P-SHG and PS-OCM. (A) System diagram showing P-SHG beam path in blue and PS-OCM in green. (B) P-SHG numerical model for quantitative analysis of collagen fibers. The SHG intensities are measured with varying excitation polarizations. In-plane fiber angles, SHG anisotropy, and helix angles can be then derived. (C) Co-registered P-SHG and PS -OCM images of a piece of rat tail tendon showing the multidimensional analyses quantifying linear scattering properties (phase retardation, birefringence, and optical axis) and SHG-derived properties of the collagen fibers.
SHG has been a powerful and non-invasive tool to visualize the microstructures of collagen and other fibrous structures [22]. While many quantitative analysis methods have been based on the morphology of collagen fibers, polarization-sensitive SHG (P-SHG) is an evolving technique that can characterize collagen using biophysical models of the second-order nonlinear susceptibility tensor, which has been demonstrated to reveal the fiber orientation, anisotropy, and even the molecular structure of collagen in terms of helix pitch angle [23]. On the other hand, polarization-sensitive optical coherence tomography and microscopy (PS-OCM) are known for quantitatively measuring tissue birefringence using the interference between the scattered light from the tissue sample and the reference beams of two orthogonal polarizations [12]. Figure 3 shows a summary of this microscope in terms of its optics design, biophysical model, and imaging results. This combined polarization-sensitive optical imaging platform can take advantage of both techniques to reveal both the linear scattering properties and second-order nonlinear susceptibility of collagen. It also applies to many other fibrous biological elements, including the cardiomyocytes, which can bring the VinUni students more insights in their research.
Aim 4: Machine learning approaches for identifying the disease stage and evaluating the medication effectiveness (Dr. Hieu Pham and Prof. Wray Buntine, Prof. Boppart, Prof. Anastasio)
Key challenges in detecting pathogens and identifying their stage in polarized light images are the sensitive detection of biological structures in the background and the transformation of the detected structures into measurements [24]. To overcome these challenges, a machine learning-assisted analysis pipeline will be developed for the identification of disease stage and the evaluation of medication effectiveness. The analysis pipeline will follow a multi-step data processing scheme (Fig. 4). Image preprocessing techniques (e.g., denoising, contrast enhancement, deconvolution) will be applied to improve the identification of biological structures in 2-D polarized images. Sarcomere structures will be then segmented using deep neural networks (e.g., U-Net and its variants [25]) (Fig. 5). Recent advances in deep learning enable efficient and accurate segmentation with reduced efforts on manual annotation and model retraining [26] After segmentation, a diverse set of features will be extracted, including sarcomere length, myofibril density, retardance, and orientation [27]. Feature engineering techniques will be applied to the raw measurements for data cleaning, batch-effect correction, and redundant feature removal. Based on the processed measurements, downstream analysis (e.g., clustering and classification)
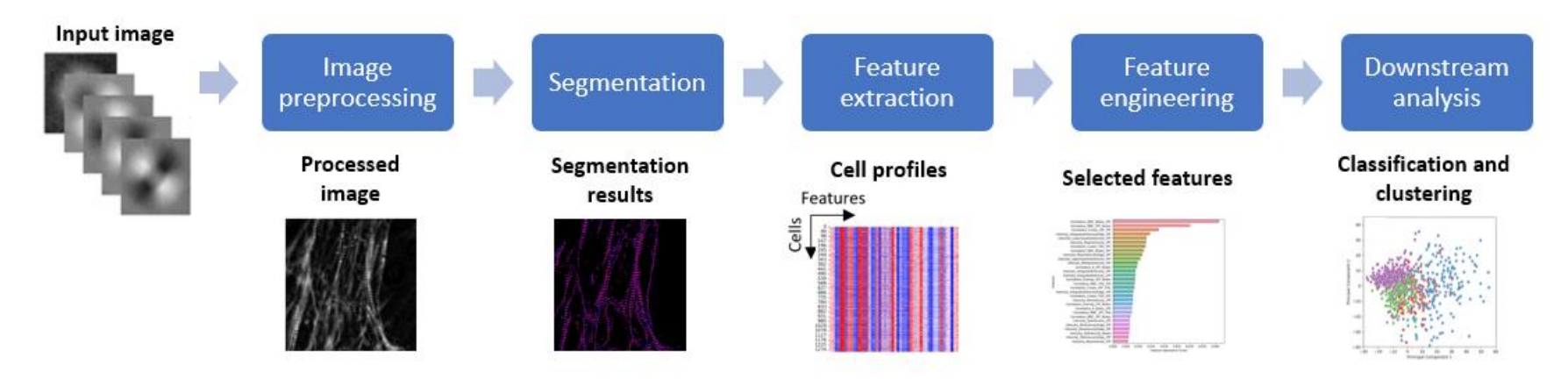
Figure 4. Machine learning-assisted analysis pipeline for the identification of disease stage and the evaluation of medication effectiveness based on 2-D polarized images.
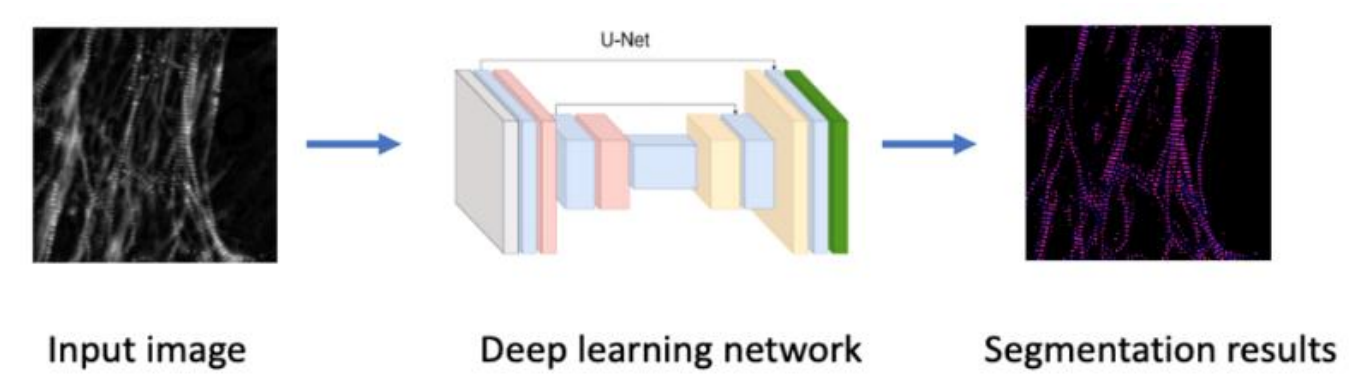
Figure 5. Segmentation of sarcomere structures using deep neural networks. Segmented sarcomere structures will be analyzed, and measurements (including sarcomere length, myofibril density, retardance, and orientation) using image processing techniques.
will be conducted to identify subpopulations and classify cells in different treatment groups. To gain insights into salient features, statistical analysis and feature importance ranking techniques (e.g., permutation importance, Mean Decrease in Impurity) will be utilized to inform the feature importance for the classification of disease stages and the medication effectiveness. In addition, end-to-end deep learning-based approaches will be explored to extract meaningful information directly from LC-PolScope images (Fig. 6). Without requiring hand-engineered features, deep learning models (e.g., convolutional neural networks) can be trained to explore medication effectiveness-related patterns without human interference [28].
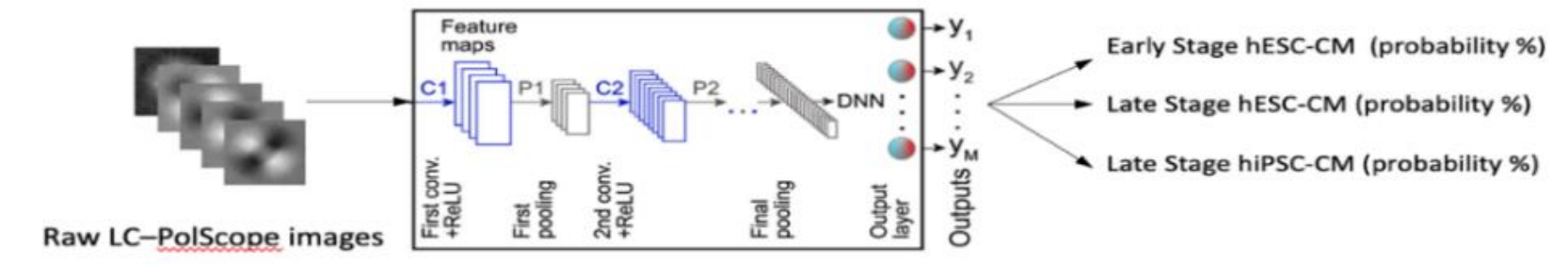
Figure 6. A deep convolutional network will be designed to classify the disease stages from the raw LC-PolScope.
To gain insight into the deep learning models, model interpretation techniques, including latent space visualization, saliency maps, and class activation maps, will be leveraged to verify model reliability and to inspire the discovery of optical signatures for disease stages and medication effectiveness [29]. These analytical approaches allow us to track developmental changes in the contractile properties of hESC-CMs and hiPSC-CMs and can be applied to many other diseases. Our research team plans to develop open-source Python software to detect and segment sarcomere structures and make it publicly available to the research community.
Summary
In summary, we propose implementing an imaging setup that enables the precise detection of small changes in the structures or dynamics of the cardiomyocyte cells under medication treatment to evaluate their cytotoxicity and efficacy, which could have broad impact on human health and wellness. Our long-term goal is to create a portable imaging setup for biomedical applications. We also explore machine learning approaches to identify and evaluate the effectiveness of the medications.
Training and mentoring plan
Students from VinUni will be recruited from different scientific backgrounds and mentored by both VinUni and UIUC PIs. Monthly remote meetings (using Zoom or Teams) will be used to follow the research progress of students and their professional development. Currently, VinUni has two candidates for the Ph.D. program (Hung Nguyen and Nhan Luong) who graduated from universities abroad and are research assistants for PIs. The results from this project will be shared openly among all investigators and researchers involved but will also be shared widely with the entire scientific, medical, and public communities. Research results will be published in peer-reviewed journals and presented publicly at national scientific and medical conferences, and investigators will share results with the public press. Optical system designs and the related software algorithms will be available through collaborative research agreements to facilitate the broad use and expanded development of our optical imaging approach.
Future development and collaboration
This project can be extended to obtain external funding from VinIF. The potential directions include using the polarized light imaging technique and AI approach developed in this project to evaluate at the cellular level the effectiveness of new medications in cancer treatments and/or infectious diseases. This research will significantly extend the collaboration between VinUni, Vinmec, and UIUC.
References
- Tani, T., et al., Postnatal structural development of mammalian basilar membrane provides anatomical basis for the maturation of tonotopic maps and frequency tuning. Scientific reports, 2021. 11(1): p. 1-12.
- DeMay B.S., et al., Septin filaments exhibit a dynamic, paired organization that is conserved from yeast to mammals. Journal of Cell Biology, 193(6):1065-1081, 2011.
- Shribak M., Oldenbourg R, Techniques for fast and sensitive measurements of two-dimensional birefringence distributions. Applied Optics, 42(16):3009-3017, 2003.
- Lewis W, et al., Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nature Reviews, 2(10):812-822, 2003.
- Poller W, et al., High incidence of cardiac dysfunction and response to antiviral treatment in patients with chronic hepatitis C virus infection. Clinical Research in Cardiology, 106(7):551-556, 2017.
- Vitiello A, Colchicine and SARS-CoV-2: management of the hyperinflammatory state. Respiratory Medicine, 178:106322, 2021.
- Choi SW, et al., Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antiviral Research, 184:104955, 2020.
- Jung SY, et al., Cardiovascular events and safety outcomes associated with remdesivir using a World Health Organization international pharmacovigilance database. Clinical and Translational Science, 15(2):501-513, 2022.
- Lundy SD, et al., Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells and Development, 22(14):1991-2002, 2013.
- Ralston TS, et al., Interferometric synthetic aperture microscopy. Nature Physics, 3(2):129-134, 2007.
- Nguyen FT, et al., Intraoperative evaluation of breast tumor margins with optical coherence tomography. Cancer Research, 69(22):8790-8796, 2009.
- South FA, et al., Differentiation of ex vivo human breast tissue using polarization-sensitive optical coherence tomography. Biomedical Optics Express, 5(10):3417-3426, 2014.
- Jung W, et al., Handheld optical coherence tomography scanner for primary care diagnostics. IEEE Transactions on Biomedical Engineering, 58(3):741-744, 2010.
- Tu H, et al., Stain-free histopathology by programmable supercontinuum pulses. Nature Photonics, 10(8):534-540, 2016.
- You S, et al., Intravital imaging by simultaneous label-free autofluorescence-multiharmonic microscopy. Nature Communications, 9(1):1-9, 2018.
- Sun Y, et al., Intraoperative visualization of the tumor microenvironment and quantification of extracellular vesicles by label-free nonlinear imaging. Science Advances, 4(12):eaau5603, 2018.
- Bower AJ, et al., High-speed imaging of transient metabolic dynamics using two-photon fluorescence lifetime imaging microscopy. Optica, 5(10):1290-1296, 2018.
- Jones GW, et al., High-spectral-resolution coherent anti-Stokes Raman scattering with interferometrically detected broadband chirped pulses. Optics Letters, 31(10):1543-1545, 2006.
- Mukherjee P, et al., Differential uptake of antisense oligonucleotides in mouse hepatocytes and macrophages revealed by simultaneous two-photon excited fluorescence and coherent Raman imaging. Nucleic Acid Therapeutics, 32(3):163-176, 2022.
- Iyer RR, et al., Label-free metabolic and structural profiling of dynamic biological samples using multimodal optical microscopy with sensorless adaptive optics. Scientific Reports, 12(1):1-15, 2022.
- Yang L, et al., Combining linear and nonlinear polarization-sensitive imaging modalities for enhanced characterizations of collagen, Biophotonics Congress: Biomedical Optics 2022, MW3A.1, 2022.
- Provenzano PP, et al., Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Medicine, 4(1):1-15, 2006.
- Mazumder N, et al., Polarization resolved second harmonic microscopy. Methods, 128:105-118, 2017.
- Guo, S.-M, et al., Revealing architectural order with quantitative label-free imaging and deep learning. Elife, 9:e55502, 2020.
- Siddique N, et al., U-net and its variants for medical image segmentation: A review of theory and applications. IEEE Access, 9: 82031-82057, 2021.
- Asgari T, et al., Deep semantic segmentation of natural and medical images: a review. Artificial Intelligence Review 54.1:137-178, 2021.
- Maragos P, Differential morphology and image processing. IEEE Transactions on Image Processing, 5(6):922-937, 1996.
- Rawat W, Zenghui Wang, Deep convolutional neural networks for image classification: A comprehensive review. Neural Computation 29.9:2352-2449, 2017.
- Adadi, A, Mohammed B, Peeking inside the black box: a survey on explainable artificial intelligence (XAI). IEEE access 6: 52138-52160, 2018.
