Development of Point-Of-Care Devices to Predict Dengue Infection Status and to Detect Sepsis Biomarkers Principal
Significance of Dengue virus:
Dengue virus (DV), a globally distributed flavivirus with 4 distinct serotypes (DV-1,-2,-3,-4) that is transmitted mainly by the urban-dwelling Aedes aegypti and Ae. albopictus mosquitoes, is fully adapted to humans and does not require an intermediary animal reservoir host 1,2. Found in over 100 tropical and subtropical countries, DV represents a significant threat to global public health. Approximately 2.5-3 billion people reside in DV-endemic areas, of whom 100-200 million individuals will be infected and around 30K patients will succumb to the disease, annually1. In Vietnam, dengue is prevalent and occurs year-round, with peak transmission in the warmer rainy season (June to October)3,4. Every year an average of 80-100K cases of dengue are registered, of which dozens are fatal. Today, dengue is hyperendemic in the southern region and central coast, and in densely populated hubs4. In some cases, dengue infection is asymptomatic. Those with symptoms get ill between 4-7 days after receiving an infectious mosquito bite. The infection is characterized by flu-like symptoms5. Although there is no specific treatment for either uncomplicated or severe dengue (there are no antivirals available), early detection and access to proper medical care reduce mortality rates to <1%3,5. The illness may progress to Dengue Hemorrhagic Fever (DHF), and high fever can last from 2-7 days. Complications can lead to circulatory system failure and shock (known as Dengue Shock Syndrome), which can be fatal5,6. Traditional tests for dengue diagnosis include molecular-based assays (Reverse transcription polymerase chain reaction, RT-PCR) and serologic tests6,7. The latter detect DV-specific immunoglobulin (Ig)M and neutralizing antibodies, which typically develop toward the end of the first week of illness. A patient with a positive IgM test result is classified as having a presumptive, recent dengue infection. The tests available are rapid but labor-intensive and relatively costly6,7. RT-PCR, meanwhile, requires virus isolation, and remains an expensive, highly technical and time-consuming method, thereby making it incompatible in regions with poor healthcare infrastructure.
Significance of Sepsis: Worldwide, over 30 million people are affected by sepsis annually (about 27% die)8. In U.S. hospitals, sepsis is the leading cause of death9, with more than 1.7 million adults developing sepsis every year (nearly 270K die in 2014)10. This mortality rate surpasses the combined mortality rate of prostate cancer, breast cancer and AIDS11. Moreover, sepsis leads all causes for hospital readmissions, with 19% of people hospitalized with sepsis needing readmissions within 30 days12. Consequently, sepsis is the #1 economic burden of hospitalization in the U.S, costing more than $27 billion each year13. Dominant factors underlying these surprisingly grim numbers include a lack of tools enabling accurate, rapid diagnosis of early sepsis, a critical step for risk stratification and determination of appropriate treatment strategies14. Likewise, Vietnam is still struggling to provide either enough resources or adequate diagnostic and treatment strategies for patients with sepsis and septic shock in both local and central settings, where the initiation of treatment in patients with sepsis is often delayed, including the administration of antibiotics15. Sepsis patients typically progress through a pro-inflammatory stage (Systemic Inflammatory Response Syndrome, SIRS), through a hybrid stage of pro/anti-inflammatory, and finally through an anti-inflammatory stage (Compensatory Anti-Inflammatory Response Syndrome, CARS)16. Each of these stages requires drastically different therapeutic intervention, but currently no accurate technique exists at the point-of-care (POC) to determine an accurate status of sepsis. Therefore, a method to quickly and accurately screen for sepsis in the primary care environment could drastically reduce undiagnosed cases and delays of appropriate treatment, while dramatically increasing survival rates and saving healthcare systems billions of dollars around the world. A diagnostic platform to identify patients at risk for sepsis in primary care, ambulances, or nursing homes allows opportune therapeutic intervention from healthcare providers. They can properly treat patients with antibiotics or hospitalize high risk cases for further treatment, instead of sending home undiagnosed cases that lead to readmission with higher mortality rates.
Intellectual Merit: In order to significantly contribute both to the early diagnosis and prediction of dengue infection status, and to the early diagnosis of sepsis, we propose to develop point-of-care microfluidic approaches to detect multiple dengue or sepsis biomarkers (nucleic acids, cells, and proteins) from the same sample of whole blood. Aware of the different serotypes of dengue and the sensitivity required, we will use multiplexed optical and electrical techniques of high sensitivity. Likewise, we will not only test for the presence of the virus by detecting nucleic acids, but also other biomarkers that can provide relevant information about a patient’s condition. In particular, we will also analyze the white blood cells count (WBC) and the presence of DV-specific IgM antibodies. Dengue is known to cause a decrease in the WBC (leukopenia; <5K cells/mm3) and platelets (thrombocytopenia; <150K cells/mm3)17. Furthermore, it has been suggested that the WBC count taken at the time hospital admission of a patient is a good indicator of the length of their stay (i.e. their recovery period)17. On the other hand, anti-DV IgM positivity is indicative of presumptive, recent dengue virus infection. Testing for DV-specific IgM combined with a nucleic acid amplification test (NAAT) generally provides a diagnostic result during the first 1-7 days of illness7. On the other hand, in the case of sepsis, its current definition (Sepsis-3 definition) defines the disease as a life-threatening organ dysfunction caused by a dysregulated host response to infection18. This definition underscores the requirements for both pathogen detection and information about the personalized state of the immune system of the patient. Consequently, recent diagnostic methods involve cellular biomarkers 14. Combinatorial detection of WBC, CD64 expression on neutrophils (nCD64), procalcitonin (PCT), C-Reactive Protein (CRP) and Interleukin 6 (IL-6) and other mediators will lead to accurate fingerprinting of sepsis, allowing early diagnosis and accurate stratification of the risks 14,19-23. Therefore, POC testing of these dengue and sepsis biomarkers could accelerate the clinical decision for early detection of dengue and sepsis, respectively. Importantly, we plan to demonstrate our approaches as global health solutions to make our technologies achievable to historically underserved populations in Vietnam and other low-income countries by reducing the existing gaps of required infrastructure and high cost.
Research Plan:
Platform 1: The first proposed platform is a microfluidic device for the simultaneous optical detection of DV RNA and/or dengue virus IgM antibodies from the same drop of whole blood (~20 µL). For the detection of DV RNA we will use loop-mediated isothermal amplification (LAMP) 24. LAMP is a one-step amplification reaction that amplifies the target nucleic acid sequence with high specificity and sensitivity under isothermal conditions and has a sensitivity comparable to traditional PCR25. The advantages of using LAMP over PCR for targeting sequences directly from whole blood are:
a) its robustness against inhibitors in whole blood such as cellular debris that inhibit a PCR reaction [26]; b) using 4-6 primers which identify 6-8 regions on the template for amplification, making it more specific than PCR 24; and c) the isothermal nature of the LAMP procedure removes the need for expensive commercial thermocyclers. Our team of researchers has recognized experience in the development of LAMP assays and their use with POC devices 27-29. In this device we initially mix the whole blood sample with lysis buffer. In case of a positive sample, virus particles are chemically lysed, and the RNA is released. Part of the lysed sample containing RNA reaches the top of the device (Figure 1) where it is mixed with the LAMP reagents and specific primers. Primers against the 4 DV serotypes are pre-spotted in four independent detection regions 27. In case of a positive sample, one of the detection regions will light up and the serotype will be identified using a smartphone-based reader, such as the ones we have recently developed 28,29. The reader will provide the lighting and temperature conditions necessary for successful isothermal amplification. Simultaneously, we will interrogate the sample to detect the presence of anti-DV IgM using a microfluidic capture chamber, such as the ones we developed before30-32. The capture chamber will be pre-functionalized with non-structural protein 1 (NS1), a highly conserved DV antigen, which will allow the specific capture of available IgM. After initial mixing of the whole blood sample with lysis buffer, all cells are lysed. In case of IgM in the sample, part of the lysed sample containing IgM reaches the capture chamber (bottom of the device, Figure 1), and is specifically captured. After that, we will tag with fluorescent anti-human-IgM to optically detect the anti-DV IgM. Fluorescence will be detected using the smartphone-based reader.
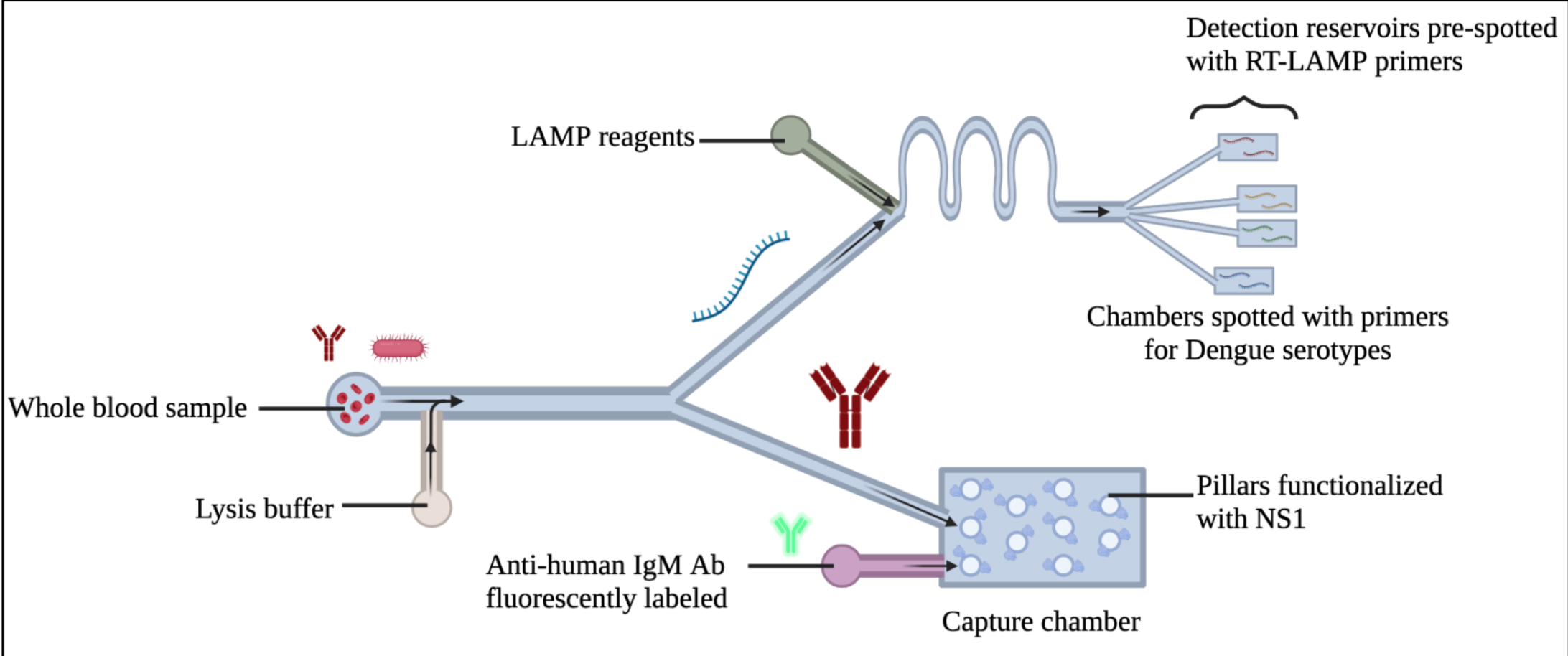
Figure 1. Schematic of Platform 1. Optical detection of dengue biomarkers.
Platform 2: The second proposed tool is a microfluidic device for the simultaneous electrical detection of DV RNA and WBC counts from the same drop of whole blood (~20 μL). In order to achieve high-sensitivity detection of DV RNA we will use a crumpled graphene field-effect transistor (cgFETs) array. Crumpled graphene is based on the nanoscale bending of 2D graphene in 3-dimensions, which results in unprecedented sensitivity. This property is due to modulation of the Debye length and the opening of the band-gap in graphene due to its bending. Using this technology we have recently shown the ability to fabricate crumpled gFET devices and their application for ultra-high sensitivity detection of proteins and nucleic acids. In this device, we will fabricate an array of four cgFETs, each of which will be functionalized with a different probe against one of the 4 DV serotypes (Figure 2). For the WBC count we will use cell counter electrodes that we developed previously. In this device, we mix the whole blood sample with lysis buffer to first lyse red blood cells (RBC). WBC are then counted in the first part of the device (Figure 2), before lysis of all cells and virus, if any, is completed. If present, virus particles are chemically lysed, and RNA is released. Probes against the 4 DV serotypes are pre-functionalized in four independent cgFETs. When a positive sample reaches the second part of the device (Figure 2)., one of the cgFETs will show a shift in the Dirac point (I-V curve).
Using this technology we have recently shown the ability to fabricate crumpled gFET devices and their application for ultra-high sensitivity detection of proteins and nucleic acids 33,34. In this device, we will fabricate an array of four cgFETs, each of which will be functionalized with a different probe against one of the 4 DV serotypes (Figure 2). For the WBC count we will use cell counter electrodes that we developed previously 30,35. In this device, we mix the whole blood sample with lysis buffer to first lyse red blood cells (RBC). WBC are then counted in the first part of the device (Figure 2), before lysis of all cells and virus, if any, is completed. If present, virus particles are chemically lysed, and RNA is released. Probes against the 4 DV serotypes are pre-functionalized in four independent cgFETs. When a positive sample reaches the second part of the device (Figure 2)., one of the cgFETs will show a shift in the Dirac point (I-V curve).
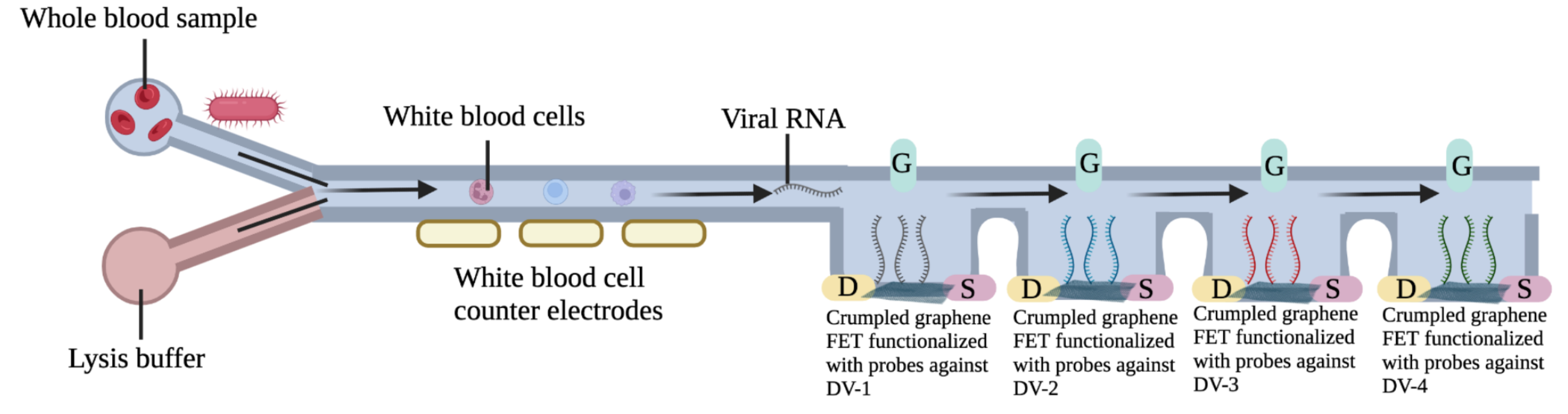
Figure 2: Schematic of Platform 2. Electrical detection of dengue biomarkers.
Platform 3: The third platform is a powerful technique to replace the use of blood culture for pathogen detection. We plan to address the lack of sensitivity and the slow process for pathogen identification by demonstrating the feasibility of a new protocol to achieve the low pathogen detection (1 -3 CFU/mL for bacteria and fungi) from 5 mL of whole blood, and in less than 2 h., via our in-house developed bi-phasic approach 36. We will develop the new generation of our bi-phasic reaction as the enabling method to improve the sensitivity of amplification reactions from large volumes of whole blood. We will characterize the reaction for 5 pathogens with higher occurrence rate at Vietnam hospital. We will spike each bacterium or fungi of interest (e.g. Staphylococcus aureus, E. coli, Klebsiella pneumoniae, Candida sp., and Streptococcus) in 5mL of whole blood from healthy donors, and then split the total volume in 5-1mL aliquots to develop the assays for the 5 proposed pathogen.
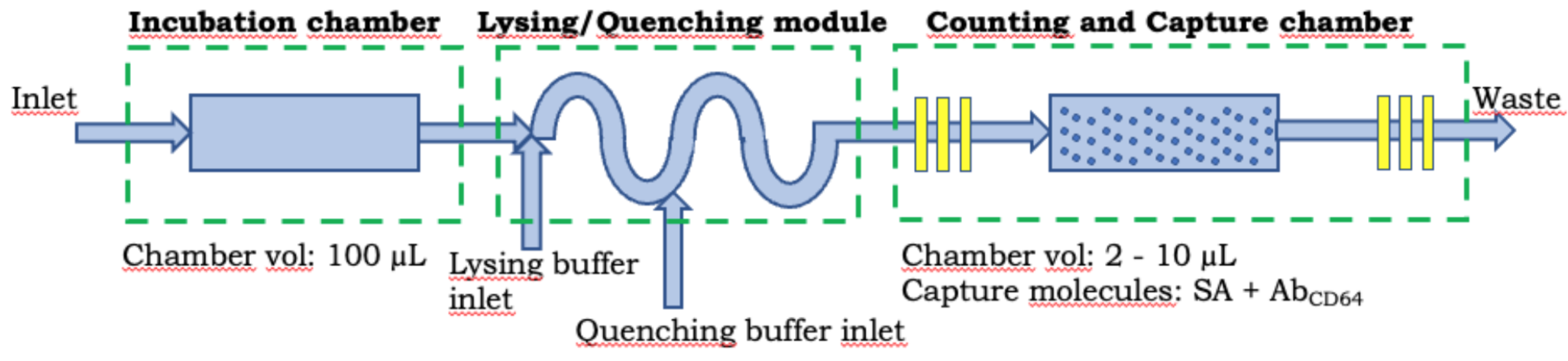
Figure 3: Schematic of Platform 4. Electrical detection of dengue biomarkers.
Platform 4: This is a microfluidic platform for measuring cell-surface proteins and plasma proteins from the same sample of whole blood. This platform will address the lack of information about the personalized state of the patient immune system by quantifying WBC, nCD64 expression, PCT, CRP and IL-6 in order to significantly contribute to the development of the sepsis fingerprint. We expect to contribute to the early diagnosis and risk stratification of sepsis. Our approach will be based on a microfluidic differential counter technology will combine electrical coulter counting and specific capture to enable the electrical quantitation 30,31,35. The proposed technology will combine the incubation chamber, lysing/quenching module, and counting and capture chamber connected in series (Figure 3). The microfluidic capture chamber consists of a symmetrically distributed pillar array for the specific capture of sepsis biomarkers.
Specific Aims:
Aim 1: Development of off-chip assays (at UIUC). We will develop the off-chip LAMP assays for detection of pathogenic nucleic acids (4 DV serotypes and 4 bacteria), and the off-chip sandwich immunoassays for detection of protein biomarkers (DV-specific IgM and sepsis biomarkers). We will spike relevant concentrations of the biomarkers of interest in whole blood. For pathogen RNA/DNA we will target a LOD ~ 10 copies/µL.
Aim 2: Fabrication and characterization of the proposed platforms for detection of dengue biomarkers (at UIUC). Device fabrication will include PDMS-based microfluidic cartridges, integration with electrodes for WBC counting, crumpled gFET array, and smartphone-based reader. Device characterization will be performed using spiked blood samples. For the image analysis, a smartphone app will be developed to take the images, perform the analysis and report the results.
Aim 3: Low pathogen detection from whole blood using our bi-phasic technique (at UIUC): In this Aim, the feasibility of the bi-phasic mechanism to detect low concentrations (1-3 CFU/mL) of 5 pathogens with higher occurrence rate in < 2 hours we will demonstrate. We will spike each bacterium or fungi of interest (Staphylococcus aureus, E. coli, Klebsiella pneumoniae, Candida sp., and virdans Streptococcus group) in 5mL of whole blood from healthy donors, and then split the total volume in 5-1mL aliquots to develop the assays for the 5 proposed pathogen.
Aim 4: POC microfluidic biochip for WBC counts and quantification of nCD64 expression, PCT, IL-6 and CRP from whole blood (at UIUC). Multiple sepsis studies have demonstrated a high level of variation in blood cell counts, nCD64 expression, and in the concentrations of PCT, IL-6 and CRP. In this Aim we will: a) develop three sandwich immunoassays for the on-chip detection of PCT, IL-6 and CRP on the surface of the magnetic hydrogel beads (MHBs)32; b) optimize the nCD64 and MHBs capture efficiency and purity; and c) design and fabricate a microfluidic biochip for complete WBC counts, and quantification of nCD64 expression, PCT, IL-6 and CRP from the same sample of whole blood. Multiple incubation cycles could be performed to improve capture efficiency and assay sensitivity.
Aim 5: Validation of the proposed devices using clinical samples (at VinUni). After fabrication and validation of devices at UIUC, we will test them at VinUni with clinical samples (positive for DV 1-4) accessed from Vinmec and elsewhere.
Qualifications:
Our multidisciplinary project requires expertise in many fields from engineering, biology, and medicine. Such a combination will lead us to creative and high impact research. Rashid Bashir is Professor of Bioengineering, Dean College of Engineering at UIUC. Prof. Bashir has extensive experience in the development of microfluidic devices for applications in biology and medicine (>200 publications, 42 patents, and co-founded 3 companies in POC diagnostics). Andrew Taylor-Robinson is Professor of Microbiology & Immunology at VinUniversity. He is an infectious disease immunologist specializing in mosquito-borne pathogens, with over 30 years’ research experience of tropical diseases, including dengue. Enrique Valera is a Research Assistant Professor in the Department of Bioengineering at UIUC with >17 years of experience in several aspects of Bioengineering, especially focused on biosensors technologies. He has a large experience in the development of POC devices for the analysis of clinical samples for diagnosis of pathogens and biomarkers. Minh Do is Professor in Signal Processing & Data Science in the Department of Electrical and Computer Engineering at UIUC. Prof. Do has extensive experience in signal processing, computational imaging, machine perception, and data science. Phung Nam Lam is Deputy CEO of Medical Affairs at Vinmec Healthcare System, and Vice Dean of Clinical Education at College of Health Sciences.
Plan to recruit and co-advise PhD students: For this highly multidisciplinary project, we plan to recruit 5-6 PhD students for a 5-year period. These students will be co-advised by UIUC and VinUni faculty in areas of bioengineering, biology, electrical and computer engineering, and infectious diseases.
Plan on the use of $20K + $20K to support each PhD student and their project:
The support that the faculty advisors will receive will be used to cover:
- Conference registration (such as BMES, Keystone Symposia, ASTMH annual meeting);
- Cost of research visit-related travel, accommodation and subsistence between VinUni-UIUC and UIUC-VinUni;
- Fess for access to specialist laboratory facilities, for instrument use, and open-access publications;
- Cost of laboratory consumables and reagents;
- Access to clinical samples (confirmed or query dengue-positive whole blood collected through liaison with Vinmec hospitals in Hanoi, Ho Chi Minh City and elsewhere that serve recognized dengue and sepsis hotspots).
Future Extensions:
Following the collection of relevant preliminary data from this project, we expect to apply for external funding (e.g., NIH R01 or NSF grants) to support an extensive clinical validation of the working platforms by comparison against current diagnostic technology, thus sustaining this multidisciplinary UIUC-VinUni research collaboration. Likewise, in these new proposals we plan to collaborate with more VinUni faculty and to highlight the potential of our microfluidic platforms as global health solutions to make our technologies achievable to historically underserved populations by reducing the existing gaps of required infrastructure and high cost.
The ultimate goal of research supported by future external funding will be to demonstrate our platforms as a powerful technique to contribute to the early diagnosis and predict the status of dengue infection, and to the early diagnosis of sepsis. It also provides an opportunity to explore technology transfer activities (potential device manufacturing partnership with VinBiocare).
We anticipate that these devices will contribute to improving current standards of tropical infectious diseases healthcare by expanding the technologies to detect other mosquito-borne viruses such as Zika and Chikungunya, and by incorporating personalized precision medicine in arbovirus and sepsis diagnosis.
References
-
- Laboratories, M. C. Test Definition: DENGC, <https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/606371> (2021).
- Nguyen-Tien, T. et al. Risk factors of dengue fever in an urban area in Vietnam: a case-control study. BMC Public Health 21, 664 (2021).
- World-Health-Organization. Dengue in Viet Nam, <https://www.who.int/vietnam/health-topics/dengue> (2021).
- World-Mosquito-Program. Vietnam, <https://www.worldmosquitoprogram.org/en/global-progress/vietnam> (2021).
- Travellers, I. A. f. M. A. t. Vietnam General Health Risks: Dengue,<https://www.iamat.org/country/vietnam/risk/dengue> (2021).
- Yow, K.-S., Aik, J., Tan, E. Y.-M., Ng, L.-C. & Lai, Y.-L. Rapid diagnostic tests for the detection of recent dengue infections: An evaluation of six kits on clinical specimens. PLoS ONE 16, e0249602 (2021).
- Centers-for-Disease-Control-and-Prevention. Serologic Tests for Dengue Virus,<https://www.cdc.gov/dengue/healthcare-providers/testing/serologic-tests.html> (2019).
- Dugani, S. & Kissoon, N. Global advocacy needed for sepsis in children. Journal of Infection 74, S61-S65 (2017).
- Liu, V. et al. Hospital Deaths in Patients With Sepsis From 2 Independent Cohorts. JAMA 312, 90-92 (2014).
- Rhee, C. et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA 318, 1241-1249 (2017).
- Heron, M. Deaths: Final Data for 2016. National Vital Statistics Reports 67 (2018).
- Kathryn Fingar & Washington, R. Trends in Hospital Readmissions for Four High-Volume Conditions, 2009-2013. Agency for Healthcare Research and Quality, Rockville, MD. Statistical Brief #196 (2015).
- Torio, C. M. & Moore, B. J. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013. Agency for Healthcare Research and Quality, Rockville, MD HCUP Statistical Brief #204 (2016).
- Daniels, R. Surviving the first hours in sepsis: getting the basics right (an intensivist’s perspective). Journal of Antomicrobial Chemotherapy 66, ii11-ii23 (2011).
- Do, S. N. et al. Factors relating to mortality in septic patients in Vietnamese intensive care units from a subgroup analysis of MOSAICS II study. Scientifc Reports 11, 18924 (2021).
- Faix, J. D. Biomarkers of Sepsis. Critical Reviews in Clinical Laboratory Sciences 50, 23-26 (2013).
- Rao, A. A., Raaju R. U, S. G. & Menon, S. Dengue Fever: Prognostic Insights From a Complete Blood Count. Cureus12, e11594 (2020).
- Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801-810 (2016).
- Su, L. et al. Value of soluble TREM-1, procalcitonin, and C-reactive protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: a prospective cohort study. BMC Infectious Diseases 12 (2012).
- Li, S. et al. Neutrophil CD64 expression as a biomarker in the early diagnosis of bacterial infection: a meta-analysis. International Journal of Infectious Diseases 17, e12-e23 (2013).
- Liesenfeld, O., Lehman, L., Hunfeld, K.-P. & Kost, G. Molecular Diagnosis of Sepsis: New Aspects and Recent Developments. European Journal of Microbiology and Immunology 4, 1-25 (2014).
- Dimoula, A. et al. Serial Determinations of Neutrophil CD64 Expression for the Diagnosis and Monitoring of Sepsis in Critically Ill Patients. Clinical Infectious Diseases 58, 820-829 (2014).
- Cohen, J. et al. Sepsis: a roadmap for future research. The Lancet Infectious Diseases Commission 15, 581-614 (2015).
- Notomi, T. et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Research 28 (2000).
- Curtis, K. A., Rudolph, D. L. & Owen, S. M. Rapid detection of HIV-1 by reverse-transcription, loop-mediated isothermal amplification (RT-LAMP). Journal of Virological Methods 151, 264-270 (2008).
- Damhorst, G. L. et al. Smartphone-Imaged HIV-1 Reverse-Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) on a Chip from Whole Blood. Engineering 1, 324-335 (2015).
- Ganguli, A. et al. Hands-free smartphone-based diagnostics for simultaneous detection of Zika, Chikungunya, and Dengue at point-of-care. Biomedical Microdevices 19, 73 (2017).
- Ganguli, A. et al. Rapid isothermal amplification and portable detection system for SARS-CoV-2. PNAS 117, 22727-22735, doi:https://doi.org/10.1073/pnas.2014739117 (2020).
- Berger, J. et al. Portable Pathogen Diagnostics Using Microfluidic Cartridges Made from Continuous Liquid Interface Production Additive Manufacturing. Anal. Chem. 93, 10048–10055 (2021).
- Hassan, U. et al. A point-of-care microfluidic biochip for quantification of CD64 expression from whole blood for sepsis stratification. Nature Communications 8, 15949 (2017).
- Valera, E. et al. A microfluidic biochip platform for electrical quantification of proteins. Lab on a Chip 18, 1461-1470 (2018).
- Cowell, T. W. et al. Rapid, multiplexed detection of biomolecules using electrically distinct hydrogel beads. Lab on a Chip 20, 2274-2283 (2020).
- Hwang, M. T. et al. Ultrasensitive detection of nucleic acids using deformed graphene channel field effect biosensors. Nature Communications 11 (2020).
- Ganguli, A. et al. High Sensitivity Graphene Field Effect Transistor-Based Detection of DNA Amplification. Adv. Funct. Mater 30 (2020).
- Watkins, N. N. et al. Microfluidic CD4+ and CD8+ T Lymphocyte Counters for Point-of-Care HIV Diagnostics Using Whole Blood. Science Translational Medicine 5 (2013).
- Mostafa, A. et al. Culture-free biphasic approach for sensitive detection of Escherichia coli O157:H7 from beef samples. Biotechnology and Bioengineering 118, 4516 (2021).
