Detection and quantitation of cancer ctDNA and miRNA for point of care lung cancer therapy selection
Budget: $260K in total ($130K for each site); including $20K for each PhD student (5 PhD students), and $10K for a postdoc at both institutions: UIUC and VinUni. 5 VinUni graduate students (Electrical and Computer Engineering, Bioengineering, chemistry, life science), 1 VinUni postdoc scholar (Biochemistry/Bioengineering/Chemistry) and $20K will be reserved for traveling and other logistic activities
Abstract
While a growing arsenal of drugs is available to treat specific molecular abnormalities across cancers, therapy effectiveness can now be predicted by detecting specific genomic circulating tumor DNA (ctDNA) and exosomal micro-RNA (miRNA) in plasma. While next-generation sequencing (NGS) can provide a comprehensive readout of genomic tumor variants that may provide biological and clinical efficacy insights, its cost, complexity, and sample-to-answer time frame are not compatible with frequent, routine, point of care diagnostics. Meanwhile, currently available laboratory-based methods for quantifying strategically-selected ctDNA and miRNA biomarkers in plasma for liquid biopsy lack sensitivity, multiplexing, and workflow simplicity required for clinical needs. A genomic liquid biopsy that can be rapidly performed in a clinical setting in the timeframe of an office visit offers a compelling alternative for identifying the presence, absence, and concentration changes in circulating nucleic acid molecules whose specific base sequences represent mutations that drive cancer-associated cellular processes. Such an approach would enable therapy selection to be performed at the earliest time while facilitating more frequent remission monitoring. To address the gaps in current technology for ctDNA analysis, we will develop and rigorously validate a novel assay method called “Activate, Cleave, Capture, and Count” (AC3) that combines two innovative elements. First, we apply a recently-demonstrated photonic crystal (PC) biosensor microscopy technology with digital resolution capability for quantifying surface-captured gold nanoparticle (AuNP) tags. Second, we utilize the CRISPR/Cas system with target-specific guide RNA probes that selectively activate cleavage of ssDNA tethers linking AuNPs to a surface, generating many released AuNPs for each ctDNA molecule. The released AuNPs are subsequently captured on a PC biosensor, where they are digitally counted. Our ”amplify-then-digitize” strategy offers a compelling alternative to digital PCR-based technologies while also circumventing the limitations inherent with thermal amplification, microdroplet partitioning, and fluorescence-based detection. Based upon preliminary results for the detection of cancer-associated ctDNA, AC3 offers a detection limit of 50 zM and a measurement of mutant allele frequency of <0.001%. We also recently developed a 10-minute, ultrasensitive assay, using the same biosensor technology platform, for detection of cancer-related miRNA using nucleic acid strand displacement reactions to convert each miRNA target molecule into many AuNPs that are captured on the PC surface. The Target Recycling Amplification Process (TRAP) achieves ~0.5 aM detection limits, surpassing alternative approaches such as PCR, while offering a simple, 1-step, room temperature, enzyme-free method that can be implemented in point of care environments. Importantly, AC3 and TRAP utilize a small and inexpensive (~ $7K) detection instrument. In this project, we will apply AC3 for characterization of plasma ctDNA biomarkers across six mutations and characterize performance using spiked-in calibration standards, and in banked human plasma samples. We will use TRAP to characterize detection of four miRNA targets associated with lung cancer clinical outcomes. We will rigorously characterize the sensitivity, selectivity, and repeatability of AC3 and TRAP compared to NGS analysis.
Background and Significance
There is a strong clinical need for a technology platform that performs multiplexed, sensitive, quantitative, rapid, and longitudinal genomic readout of tumor biology through detection of nucleic acid molecules released during initial disease development, treatment, and recurrence. While Next Generation Sequencing (NGS) technologies aid the discovery of new genomic biomarkers for cancer, NGS does not provide the required sensitivity for detecting circulating tumor DNA (ctDNA)1 in plasma that identifies the presence of genetic variants when only a few target tumor gene copies exist within a mixed pool comprised of millions of non-tumor sequences in circulating free DNA (cfDNA). We seek to address an important gap in the capabilities of existing methods to enable routine point of care detection/quantification of tumor associated genes in blood prior to, during, and after treatment. Such an approach would enable more timely initial therapy selection, provide a longitudinal readout of the effects of therapy while it is underway, and enable routine post-therapy follow-up as clonal evolution emerges under selective treatment pressures. In this project, we will focus specifically upon rigorously demonstrating the performance of a novel assay approach called “Activate, Cleave, Capture, and Count” (AC3) for ultra-sensitive detection and quantification of several well-known mutations with clinical relevance for guiding initial therapy selection. Specifically, we will design, demonstrate, and validate AC3 assays for KRAS mutations in lung, colorectal, and pancreatic cancers. However, the technology developed in this project is broadly applicable to any ctDNA target sequence, through design of the nucleic acid probes that specifically recognize the base sequence of their target.
Our objective is to affordably reduce the time to treatment by improving molecular testing so that targeted therapies can be started within hours of diagnosis rather than weeks or months. We will utilize the most frequently mutated ctDNA biomarkers and cancer-related miRNA in Vietnamese patients. For example, a recent study of 350 Vietnamese patients with NSCLC showed mutations in EGFR (35.4%) and KRAS (22.6%)2. Our preliminary results show that AC3 can detect mutations in plasma more rapidly and with greater sensitivity than other liquid biopsy approaches such as droplet digital PCR (ddPCR), where we achieve 50 zM Limit of Detection (LOD) of the mutant sequence and >2,300:1 discrimination against the wildtype sequence, representing the potential for sensing 0.001-0.0001% mutant allele frequency. Importantly, the CRISPR/Cas-based assay workflow for AC3 is simple and rapid, and the digital-resolution biosensing method, utilizing photonic crystal (PC) biosensor microscopy, is portable, simple, and inexpensive. The rationale for this approach is that successful completion of our proposed aims will result in a rapid diagnostic test to detect the most common DNA mutations for which there is a targeted therapy, and for which there is clinical value in longitudinal monitoring of the same mutation during and after treatment for detection of molecular residual disease. This development would have an immediate impact on clinical practice.
Exosomal microRNAs (miRNAs) sequestered within extracellular vesicles play diverse roles in biological processes, including cell-cell communication, cell proliferation, and inflammatory response. Inappropriate miRNA release from exosomes can result in the development of cardiovascular diseases and cancers. On this basis, exosomal miRNAs are recognized as pivotal biomarkers for diagnosing cancer development and monitoring the progression of diseases and correlating therapeutic effectiveness. However, exosomal miRNAs may be present in extremely low concentrations, which is a current barrier to utilizing exosomal miRNAs as biomarkers. For exosomes isolated from cells or plasma, there can be far less than a single miRNA per exosome on average, even for the most abundant target sequences.
Traditional methods such as quantitative reverse transcription polymerase chain reaction (qRT-PCR) for miRNA have been considered as the gold standard for miRNA quantification with femtomolar limits of detection. However, the traditional qRT-PCR requires complicated enzymatic amplification and complicated primer designs. Constrained by the detection limit, no previous methods have been adopted for clinical use for exosomal miRNA detection without enzymatic target amplification. Therefore, there is an unmet need to develop an ultrasensitive and highly selective diagnostic approach without enzymatic amplification to effectively detect and quantify exosomal miRNAs.
Goals
Our overall strategy is:
- Development of an assay format for AC3 that is compatible with multiplexing and enhanced throughput, along with design and validation of synthetic guide RNA (sgRNA) probes for the most common mutations in Vietnamese lung cancer patients.
- Rigorous and stepwise characterization of the assay to meet quantitative performance metrics. Through these Aims, the project will rigorously characterize the sensitivity, selectivity, and repeatability of multiplexed AC3 and TRAP using target mutations with a high degree of clinical relevance, while providing direct comparison to a gold standard method. Our project culminates in combined evaluation of miRNA and ctDNA from plasma samples of patients, pre- and post-treatment, providing advantages over current practices using NGS.
Aim 1: To rigorously characterize multiplex AC3 repeatability, detection limit, and clinical accuracy
Aim 1A: Design and validation of sgRNA probes for the 4 most common m-ctDNA mutations in Vietnamese lung cancer patients and their corresponding wt-cfDNA sequences
Aim 1B: Determine false positive counts for blank controls and optimize assay protocol
Aim 1C: Utilizing reference standards for each m-ctDNA doped into plasma from 0.0001% to 10% mutant allele frequency, compare actual vs. measured mutant allele frequency and limit of quantitation
Aim 1D: Comparative detection between AC3 and NGS with 100 clinical plasma specimen.
Aim 2: To rigorously characterize multiplex TRAP repeatability, detection limit, and clinical accuracy
Aim 2A: Design and validation of nucleic acid strand displacement probes for 4 miRNA target molecules associated with clinical outcomes in lung cancer.
Aim 2B: Utilizing reference standards for each miRNA doped into plasma from 1 aM to 1 pM concentration, derive dose-response reference standards.
Aim 2C: Comparative detection between TRAP with plasma and DNA/RNA-Seq NGS with tissue/plasma in 100 clinical plasma specimens
Aim 3: Measure circulating miRNA and ctDNA biomarkers in plasma samples from VinMec patients. Measure potential correlations between biomarker levels and disease recurrence, utilizing the combined information from two independent biomarker classes, focusing specifically upon patients with NSCLC.
Approach
The goal of this proposal is to develop and characterize innovative and ultra-sensitive technologies that utilize novel concepts in nanotechnology to provide digital resolution counting of the low abundant target ctDNA and miRNA in plasma. Compared to existing approaches. Our preliminary results show that our method offers assays that are simple, quantitative, and sensitive enough to enable precise adjustment of therapy and detection of minimal residual disease. Further, we offer an instrumentation approach that can be implemented with a ~$7K system suitable for operation in clinics and hospitals providing point of care diagnostics. We have assembled a team of uniquely qualified multi-disciplinary faculty in the fields of oncology, cancer genomics, bioengineering, and nucleic acid engineering to achieve the initial proof-of-principle demonstration of this technology platform.
Our goal is to develop a rapid (<2 h sample-to-answer), affordable, point of care test for four of the most common mutations in non-small cell lung cancer (NSCLC), strategically selected to inform clinical decision-making for first-line chemotherapy. The successful development of rapid, point of care tests for cancer-related nucleic acid biomarkers, could have many applications. First, we envision that this test could be adopted in oncology offices and hospitals such that patients could be matched to a treatment during their initial visit with an oncologist and leave with a targeted therapy in hand. This would significantly reduce time to treatment from diagnosis and minimize disease progression associated with traditional molecular profiling. Even though the adoption of next generation sequencing into clinical practice has revolutionized our approach to targeted therapy, it has simultaneously increased how long patients wait to start treatment. Second, with the ongoing development of novel therapeutics, a rapid, point of care test could identify patients who meet molecular eligibility for clinical trials and reduce their times to start of treatment on trial. Third, a rapid, point of care test could have significant implications with the promising developments of neoadjuvant systemic therapies. Fourth, as molecular surveillance is being integrated into clinical trials and clinical practice, our testing approach could be used for the assessment of molecular residual disease. Although we currently do not have therapeutic strategies for the treatment of molecular residual disease in lung cancer, an affordable, rapid, and sensitive test could be embedded into future trials to determine its application. We expect our assay to complement traditional, whole exome sequencing or large gene panel approaches, while significantly reducing patient wait times for treatment.
Due to the limitations of PCR, CRISPR/Cas-based detection approaches are gaining traction in the field of pathogenic and cancer-related point of care diagnostics3-5. Single-effector RNA-guided nucleases, such as Cas12 can be reprogrammed with CRISPR RNAs (crRNAs) (also called “sgRNA”) to provide a platform for detection of specific DNA sequences. Upon recognition of its target sequence, activated Cas complexes immediately perform indiscriminate “collateral” cleavage (trans-endonuclease activity) of any nearby non-specific single-stranded DNA (ssDNA)6. Once the target-bound CRISPR Cas12a RNP complex activates, it begins collateral cleavage of nearby non-specific ssDNA molecules which can be labeled for in vitrodetection7. While CRISPR-based detection is advantageous over PCR because it is isothermal and highly selective, its major bottleneck is the need for a pre-reaction amplification step requiring primers and DNA polymerase before the CRISPR detection step can occur. The amplification is needed because the biosensor transduction methods used thus far do not provide sufficient sensitivity.
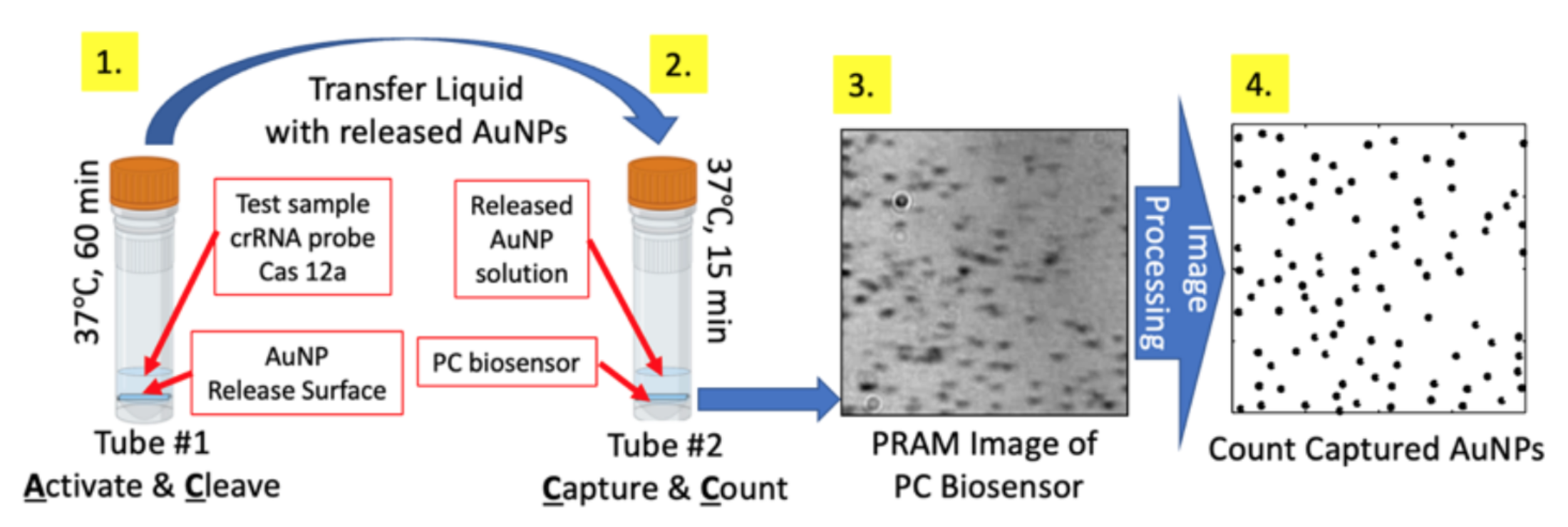
“Activate, Cleave, Capture, and Count” (AC3) for ultrasensitive ctDNA detection. AC3 utilizes an innovative form of signal amplification, in which a single target ctDNA molecule interacts with a sequence-specific sgRNA to activate one Cas12a enzyme molecule. Activation occurs in the presence of streptavidin-coated gold nanoparticles (AuNPs) that are tethered to a glass “release surface” by ssDNA (Fig. 1). The amplification mechanism occurs through rapid generation of many released AuNPs into the solution by each activated Cas12a molecule. The number of released AuNP is linearly dependent upon the target cfDNA concentration. The second key innovation within AC3 is the ability to digitally count the released streptavidin-coated AuNPs by capturing them on a biotinylated photonic crystal (PC) surface, whose resonant reflection wavelength matches the AuNP localized surface plasmon resonant (LSPR) wavelength (Fig. 3). Using a recently developed (by the Cunningham group) biosensor microscopy approach called Photonic Resonator Absorption Microscopy (PRAM)8-11, each PC-attached AuNP is clearly observed as a dark spot (Fig. 1) in an image that is generated simply by illuminating the PC with a low-intensity LED, and viewing the reflected image with a webcam-quality CMOS image sensor. The portable instrument cost is ~$7K to assemble from off-the-shelf components12. Therefore, our approach circumvents all issues associated with precise thermal cycling, microdroplet partitioning, fluorescence excitation, and fluorescence imaging that are inherent in competing PCR-based approaches, while taking advantage of the elegant simplicity and exquisite selectivity of the sgRNA probe used to activate Cas12a when the cfDNA target molecule is in a stable double-stranded initial state. The resulting workflow (Fig. 1) is simple, rapid sample-to-answer, and straightforward to multiplex for parallel readout of several cfDNA targets. Importantly, our preliminary results demonstrate the capability for 0.0001% limit of detection for mutant allele frequency, surpassing ddPCR and BEAMing, while providing quantitation over a 50 zM-50 fM dynamic range. The greater sensitivity of AC3 relative to digital PCR arises because, in ddPCR and BEAMing, one cfDNA molecule can produce only one positive event, in the form of a bright bead or droplet. In contrast, one cfDNA molecule in an AC3 assay produces many released AuNPs, which are digitally counted. The “amplify-then-digitize” approach inherently provides a high signal-to-noise in terms of true positive events versus background false-positive events, as long as AuNPs are not unintentionally released by spuriously activated Cas12a. The inherent sensitivity advantage may be used to detect mutant genes at a lower concentration, to increase multiplexing, or to reduce requirements for plasma volume.
Target Recycling Amplification Process (TRAP) for ultrasensitive miRNA detection. As shown in Fig. 2, Extracted exosomal miRNAs are placed in a PDMS reservoir applied to the PC surface along with probes linked to AuNPs. Next, a DNA linker (green) is pre-annealed with a partially complementary protector (blue) and hybridizes with DNA capture. In the presence of target miRNA (red), linker -protector are “activated” through displacement of the protector, resulting in the exposure of an Additional DNA linker sequence (toehold-2). Then Probe-modified (pink) AuNPs hybridize with the DNA linker through displacement of the miRNA, resulting in an AuNP covalently attached to the PC biosensor while the miRNA target is released for additional rounds of detection.
PC biosensors and photonic resonator absorption microscopy (PRAM) for digital resolution detection Photonic crystals (PCs) are a category of optical resonators that hold promise for digital resolution biosensing and microscopy13. A PC is a periodic arrangement of dielectric permittivity which can produce many of the same phenomena for photons that the atomic potential produces for electrons14 (Fig. 3a). By adjusting the parameters/materials of the PC, the electromagnetic fields associated with light can be manipulated to generate resonances at specific wavelengths for enhancement of light-matter interactions. Recent research by our team has shown that AuNPs with LSPR resonance that spatially and spectrally overlap with the PC’s surface-confined resonant electromagnetic field yield a 10-fold amplification of the absorption efficiency15. The synergistic coupling between the AuNP and the PC surface leads to the capability to observe individual AuNPs using a conventional inverted optical microscope, as the enhanced AuNP absorption can attenuate light that is reflected into a microscope objective.
Utilizing PC-AuNP resonant coupling, a new form of biosensor microscopy called Photonic Resonator Absorption Microscopy (PRAM) was recently demonstrated by the Cunningham group8,10,16,17. The PC is designed to provide 100% reflection efficiency at a resonant wavelength of 633 nm, at which complete interference occurs, and no light is transmitted, resulting in nearly 100% reflection efficiency (Fig. 3b). The resonant reflectance magnitude is dramatically reduced by the addition of absorbing AuNPs upon the PC surface (Fig. 3b), resulting in the ability to observe each AuNP by illuminating with light from an LED that emits at a wavelength of 633 nm ( Fig. 3c) and making images of the reflected intensity (Fig. 3d). By measuring the reflected intensity on a pixel-by-pixel basis across the PC using PRAM, images of attached AuNPs may be gathered by illuminating the structure with collimated broadband light through the transparent substrate, while the front surface of the PC is immersed in aqueous media.
The PC biosensor (Fig. 3a) is fabricated by large area holographic lithography on 8-inch diameter glass wafers using a design described in prior reports from the Cunningham Group. The device has a linear grating surface structure (period = 400 nm, depth = 120 nm), producing a resonant narrow band optical reflection, as shown in Fig. 3b. The PCs are inexpensively
manufactured by a commercial provider (Moxtek, Orem, UT) to our design specifications at a cost of <$1/test. We recently demonstrated an inexpensive and portable version of the PRAM
instrument12 that eliminates the spectrometer and the motion stage used in our initial reports. The components of the instrument are a low intensity LED, a linear polarizing filter, a microscope objective, and a webcam-quality silicon CMOS image sensor.
Plan For Training And Talent Development
Developing future talents is one of the key objectives of the project. We will work closely with the Vingroup scholarship team for advertising, interviewing, and recruiting graduate students with matching backgrounds. Based on the nature of the multi-disciplinary fields, and the time-line requirements at each stage, our plan to recruit and co-advise PhD, MS students, and postdoctoral scholars:
- 1st year: Recruit 1 PhD and 1 MS student with a biomedical/bioelectronics/physics background. The PhD student will enroll in UIUC. The Master student will enroll in VinUni (as VinUni does not yet offer a PhD in engineering); Recruit 1 postdoc in bioengineering, chemistry, or life science co-advised by VinUni and UIUC faculty.
- 2nd year: Recruit 1 PhD student in bioengineering, chemistry, or life science enrolling in UIUC.
- 3rd year: Recruit 1 PhD student in EECS enrolling in VinUni. 1 MS student will enroll in VinUni.
- 4th year: Recruit 1 PhD student with a biomedical, bioelectronics background enrolling in VinUni (Anticipating VinUni will have an engineering PhD program at that time).
- 5th year: Recruit 1 PhD student in EECS enrolling in VinUni. 1 MS student will enroll in UIUC.
Referenced Literature
- Merker, J. D. et al. Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American
- Dang, A. T. H. et al. Actionable Mutation Profiles of Non-Small Cell Lung Cancer patients from Vietnamese population. Sci Rep-Uk 10 (2020). 10.1038/s41598-020-59744-3
- Tsou, J. H., Leng, Q. & Jiang, F. A CRISPR Test for Rapidly and Sensitively Detecting Circulating EGFR Mutations. Diagnostics (Basel) 10 (2020).
4. Abudayyeh, O. O. et al. RNA targeting with CRISPR-Cas13. Nature 550, 280-284 (2017).
- Ganbaatar, U. & Liu, C. CRISPR-Based COVID-19 Testing: Toward Next-Generation Point-of-Care Diagnostics. Frontiers in Cellular and Infection Microbiology 11(2021).
- Knott, G. J. et al. Guide-bound structures of an RNA-targeting A-cleaving CRISPR-Cas13a enzyme. Nat Struct Mol Biol 24, 825-833 (2017).
- Chen, J. S. et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 360, 436-439 (2018).
- Canady, T. D. et al. Digital-resolution detection of microRNA with single-base selectivity by photonic resonator absorption microscopy. Proc Natl Acad Sci U S A116, 19362-19367 (2019).
- Che, C. et al. Activate capture and digital counting (AC + DC) assay for protein biomarker detection integrated with a self-powered microfluidic cartridge. Lab Chip 19, 3943-3953 (2019).
- Zhao, B., Che, C., Wang, W., Li, N. & Cunningham, B. T. Single-step, wash-free immunoassay for rapid quantitative analysis of serological antibody against SARS-CoV-2 by photonic resonator absorption microscopy. Talanta 225, 122004 (2021).
- Zhao, B. et al. Digital-resolution and highly sensitive detection of multiple exosomal small RNAs by DNA toehold probe-based photonic resonator absorption microscopy. Talanta 241 (2022). 10.1016/j.talanta.2022.123256
- Ghosh, S. et al. A portable photonic resonator absorption microscope for point of care digital resolution nucleic acid molecular diagnositcs. Biomedical Optics Express 12, 4637-4650 (2021).
- Zhang, Y. N., Zhao, Y. & Lv, R. Q. A review for optical sensors based on photonic crystal cavities. Sensor Actuat a-Phys 233, 374-389 (2015).
- Joannopoulos, J. D., Johnson, S. G., Winn, J. N. & Meade, R. D. Photonic Crystals: Molding the Flow of Light. 2nd edn, (Princeton University Press, 2008).
- Huang, Q. & Cunningham, B. T. Microcavity-Mediated Spectrally Tunable Amplification of Absorption in Plasmonic Nanoantennas. Nano Lett 19, 5297-5303 (2019).
- Zhuo, Y. et al. Single nanoparticle detection using photonic crystal enhanced microscopy. The Analyst 139, 1007-1015 (2014).
